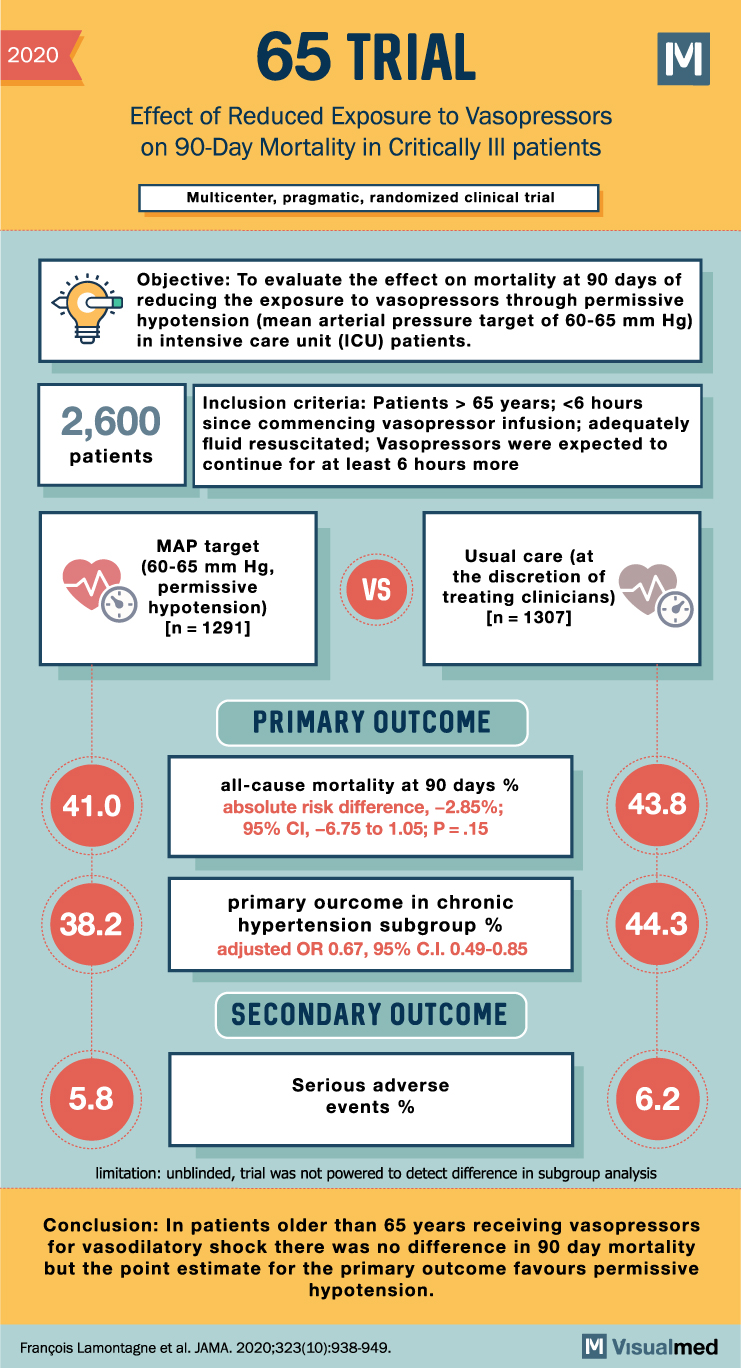
2020 65 TRIAL Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Critically III patients Multicenter, pragmatic, randomized clinical trial Objective: To evaluate the effect on mortality at 90 days of reducing the exposure to vasopressors through permissive hypotension (mean arterial pressure target of 60-65 mm Hg) in intensive care unit (ICU) patients. 2,600 patients Inclusion criteria: Patients > 65 years; <6 hours since commencing vasopressor infusion; adequately fluid resuscitated; Vasopressors were expected to continue for at least 6 hours more MAP target (60-65 mm Hg, permissive hypotension) (n = 1291] VS Usual care (at the discretion of treating clinicians) (n = 1307] PRIMARY OUTCOME 41.0 all-cause mortality at 90 days % absolute risk difference, -2.85%; 95% CI, -6.75 to 1.05; P=.15 43.8 38.2 primary ourcome in chronic hypertension subgroup % adjusted OR 0.67, 95% C.I. 0.49-0.85 44.3 SECONDARY OUTCOME 5.8 Serious adverse events % 6.2 limitation: unblinded, trial was not powered to detect difference in subgroup analysis Conclusion: In patients older than 65 years receiving vasopressors for vasodilatory shock there was no difference in 90 day mortality but the point estimate for the primary outcome favours permissive hypotension. François Lamontagne et al. JAMA. 2020;323(10):938-949.