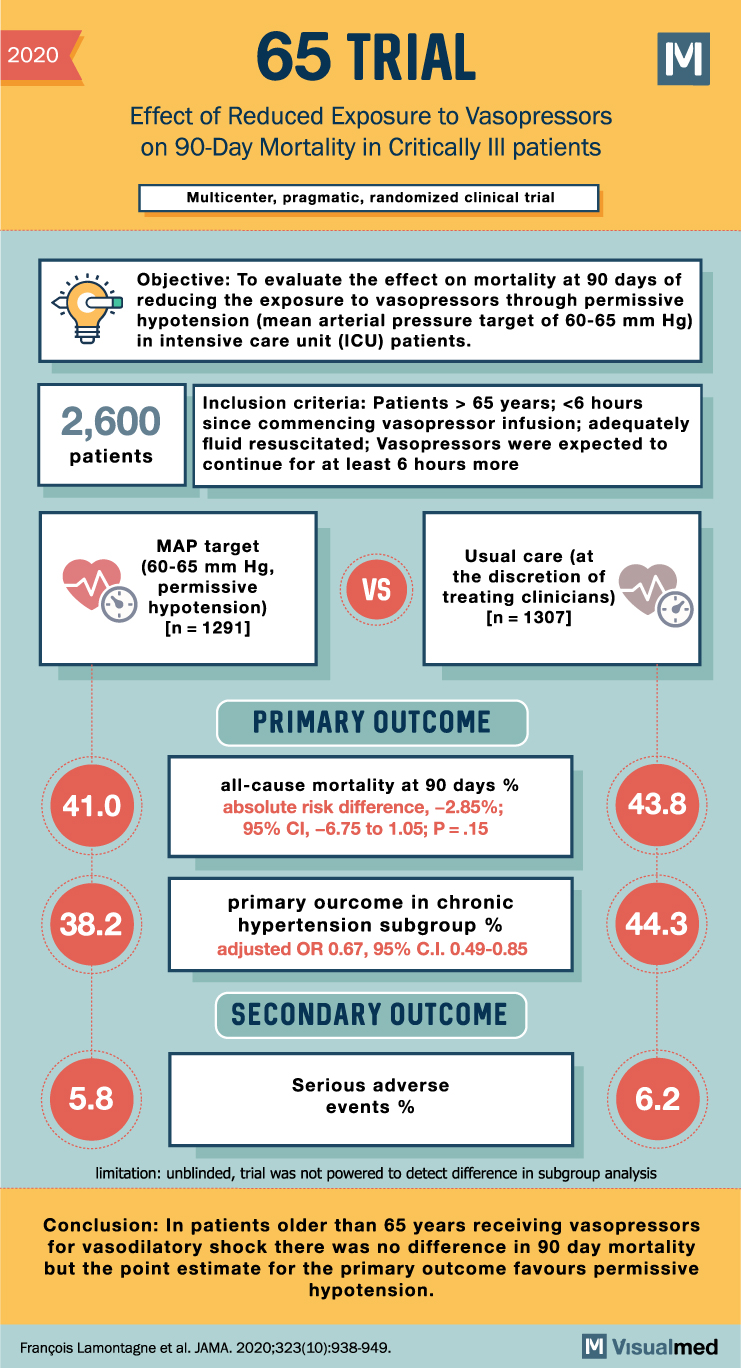
2020 65 TRIAL Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Critically III patients Multicenter, pragmatic, randomized clinical trial Objective: To evaluate the effect on mortality at 90 days of reducing the exposure to vasopressors through permissive hypotension … Read More
hypotension
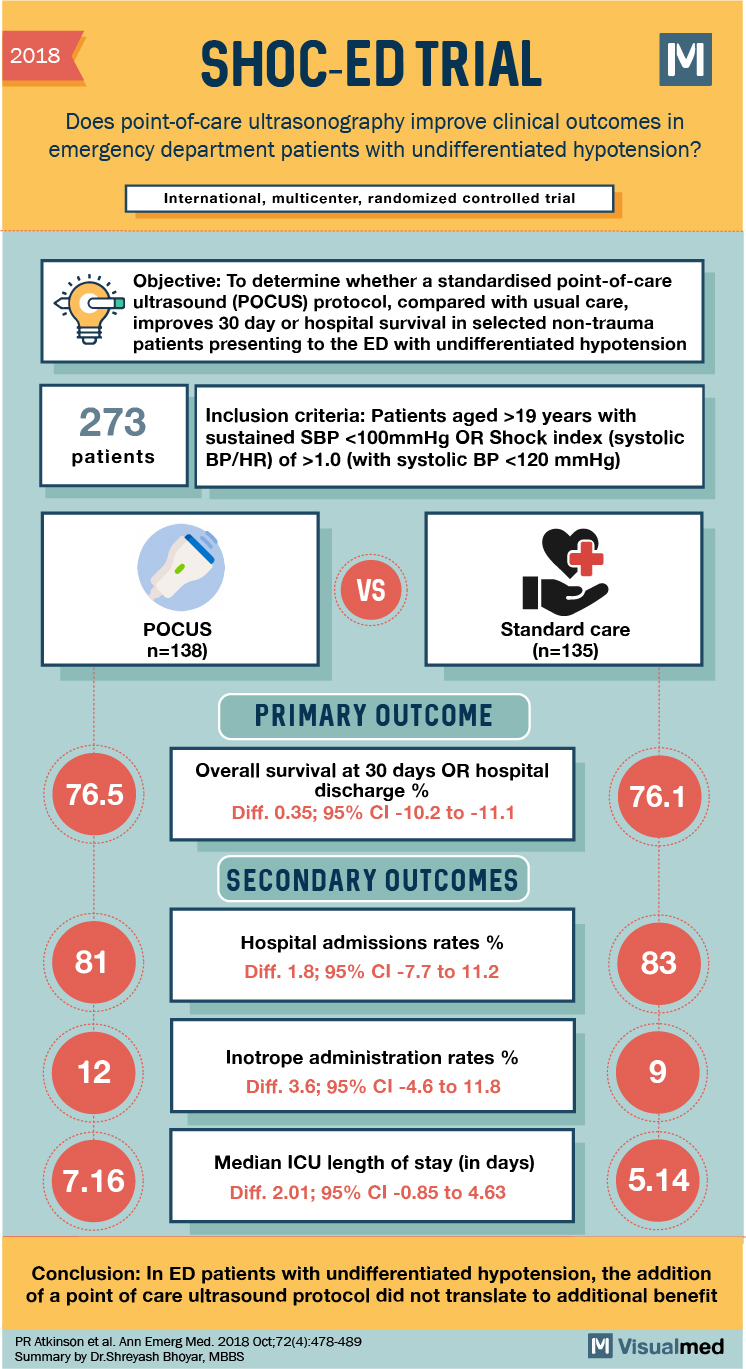
SHoC-ED Trial Summary: POCUS in ER for Hypotension
2018 SHOC-ED TRIAL M Does point-of-care ultrasonography improve clinical outcomes in emergency department patients with undifferentiated hypotension? International, multicenter, randomized controlled trial Objective: To determine whether a standardised point-of-care D ultrasound (POCUS) protocol, compared with usual care, improves 30 day … Read More
Tags: hypotension, POCUS, shock, ultrasound
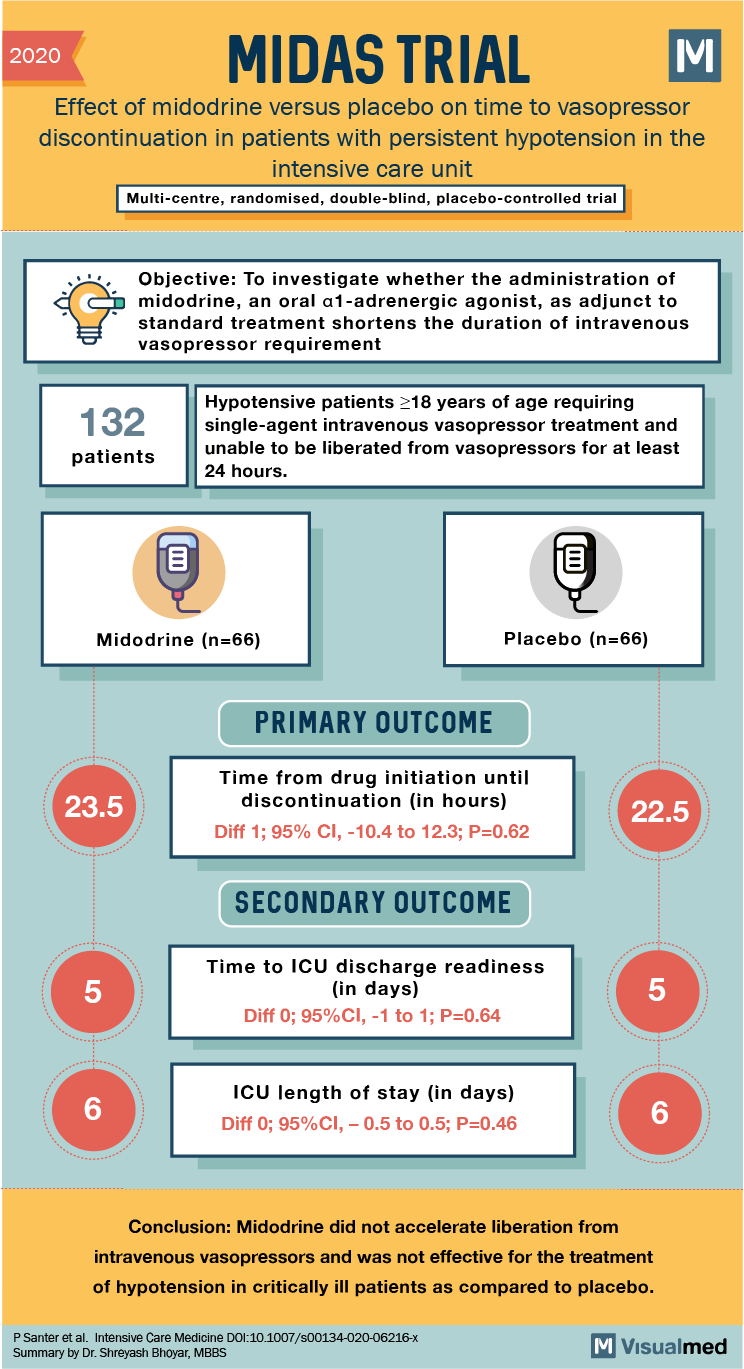
MIDAS Trial Summary: Midodrine for Vasopressor Weaning
2020 MIDAS TRIAL: Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit Multi-centre, randomised, double-blind, placebo-controlled trial Objective: To investigate whether the administration of midodrine, an oral al-adrenergic agonist, … Read More
Tags: hypotension, ICU, midodrine, placebo, shock, vasopressor