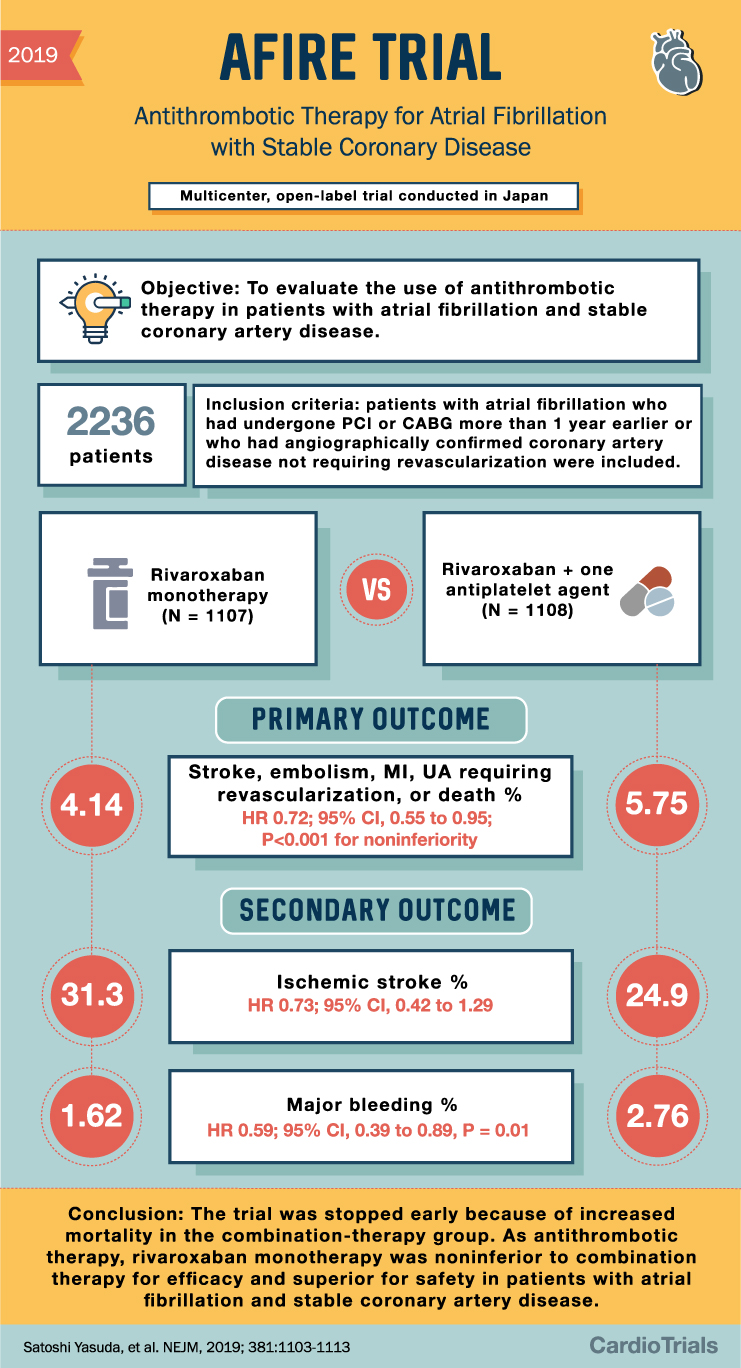
2019 AFIRE TRIAL Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease Multicenter, open-label trial conducted in Japan Objective: To evaluate the use of antithrombotic therapy in patients with atrial fibrillation and stable coronary artery disease. 2236 patients 4.14 Inclusion criteria: patients with atrial fibrillation who had undergone PCI or CABG more than 1 year earlier or who had angiographically confirmed coronary artery disease not requiring revascularization were included. Rivaroxaban monotherapy VS (N = 1107) Rivaroxaban + one antiplatelet agent (N = 1108) PRIMARY OUTCOME Stroke, embolism, MI, UA requiring revascularization, or death % HR 0.72; 95% CI, 0.55 to 0.95; P<0.001 for noninferiority SECONDARY OUTCOME 5.75 Ischemic stroke % 31.3 24.9 HR 0.73; 95% CI, 0.42 to 1.29 1.62 Major bleeding % 2.76 HR 0.59; 95% CI, 0.39 to 0.89, P = 0.01 Conclusion: The trial was stopped early because of increased mortality in the combination-therapy group. As antithrombotic therapy, rivaroxaban monotherapy was noninferior to combination therapy for efficacy and superior for safety in patients with atrial fibrillation and stable coronary artery disease. Satoshi Yasuda, et al. NEJM, 2019; 381:1103-1113