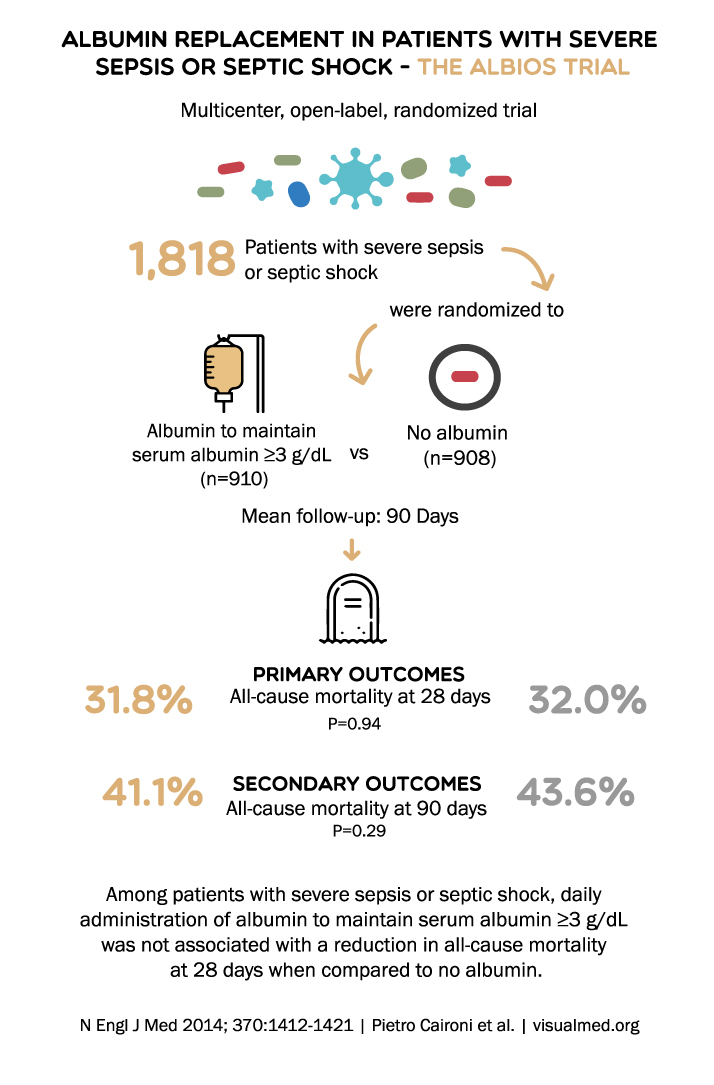
ALBUMIN REPLACEMENT IN PATIENTS WITH SEVERE SEPSIS OR SEPTIC SHOCK – THE ALBIOS TRIAL Multicenter, open-label, randomized trial Patients with severe sepsis 1,818 or septic shock were randomized to Albumin to maintain serum albumin ≥3 g/dL vs (n=910) No albumin (n=908) Mean follow-up: 90 Days PRIMARY OUTCOMES 31.8% All-cause mortality at 28 days P=0.94 41.1% SECONDARY OUTCOMES All-cause mortality at 90 days P=0.29 32.0% 43.6% Among patients with severe sepsis or septic shock, daily administration of albumin to maintain serum albumin ≥3 g/dL was not associated with a reduction in all-cause mortality at 28 days when compared to no albumin. N Engl J Med 2014; 370:1412-1421 | Pietro Caironi et al.