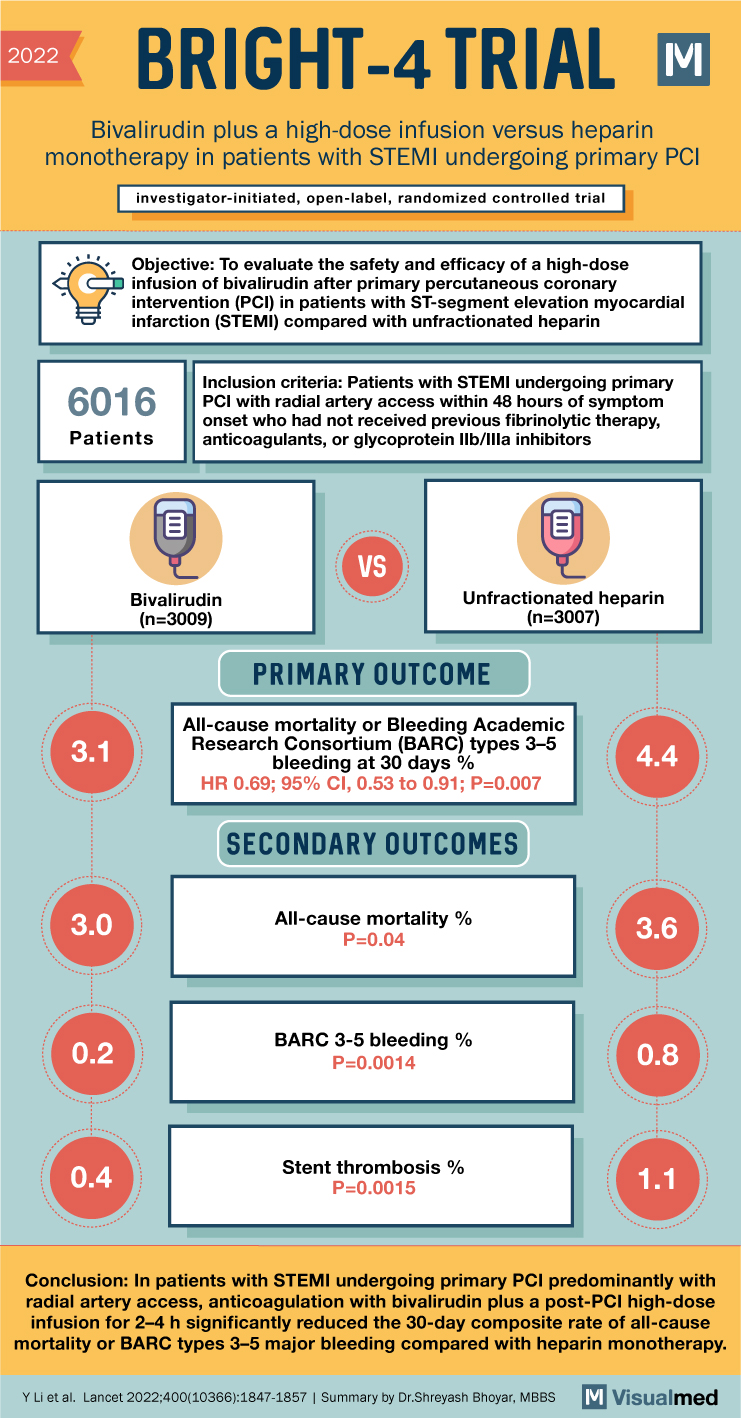
2022 BRIGHT-4 TRIAL Bivalirudin plus a high-dose infusion versus heparin monotherapy in patients with STEMI undergoing primary PCI investigator-initiated, open-label, randomized controlled trial Objective: To evaluate the safety and efficacy of a high-dose infusion of bivalirudin after primary percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI) compared with unfractionated heparin 6016 Patients Inclusion criteria: Patients with STEMI undergoing primary PCI with radial artery access within 48 hours of symptom onset who had not received previous fibrinolytic therapy, anticoagulants, or glycoprotein IIb/Illa inhibitors 3.1 9 B Bivalirudin (n=3009) VS Unfractionated heparin (n=3007) PRIMARY OUTCOME All-cause mortality or Bleeding Academic Research Consortium (BARC) types 3-5 bleeding at 30 days % HR 0.69; 95% CI, 0.53 to 0.91; P=0.007 SECONDARY OUTCOMES 4.4 3.0 All-cause mortality % P=0.04 3.6 0.2 BARC 3-5 bleeding % P=0.0014 0.8 0.4 Stent thrombosis % P=0.0015 1.1 Conclusion: In patients with STEMI undergoing primary PCI predominantly with radial artery access, anticoagulation with bivalirudin plus a post-PCI high-dose infusion for 2-4 h significantly reduced the 30-day composite rate of all-cause mortality or BARC types 3-5 major bleeding compared with heparin monotherapy. Y Li et al. Lancet 2022;400(10366):1847-1857