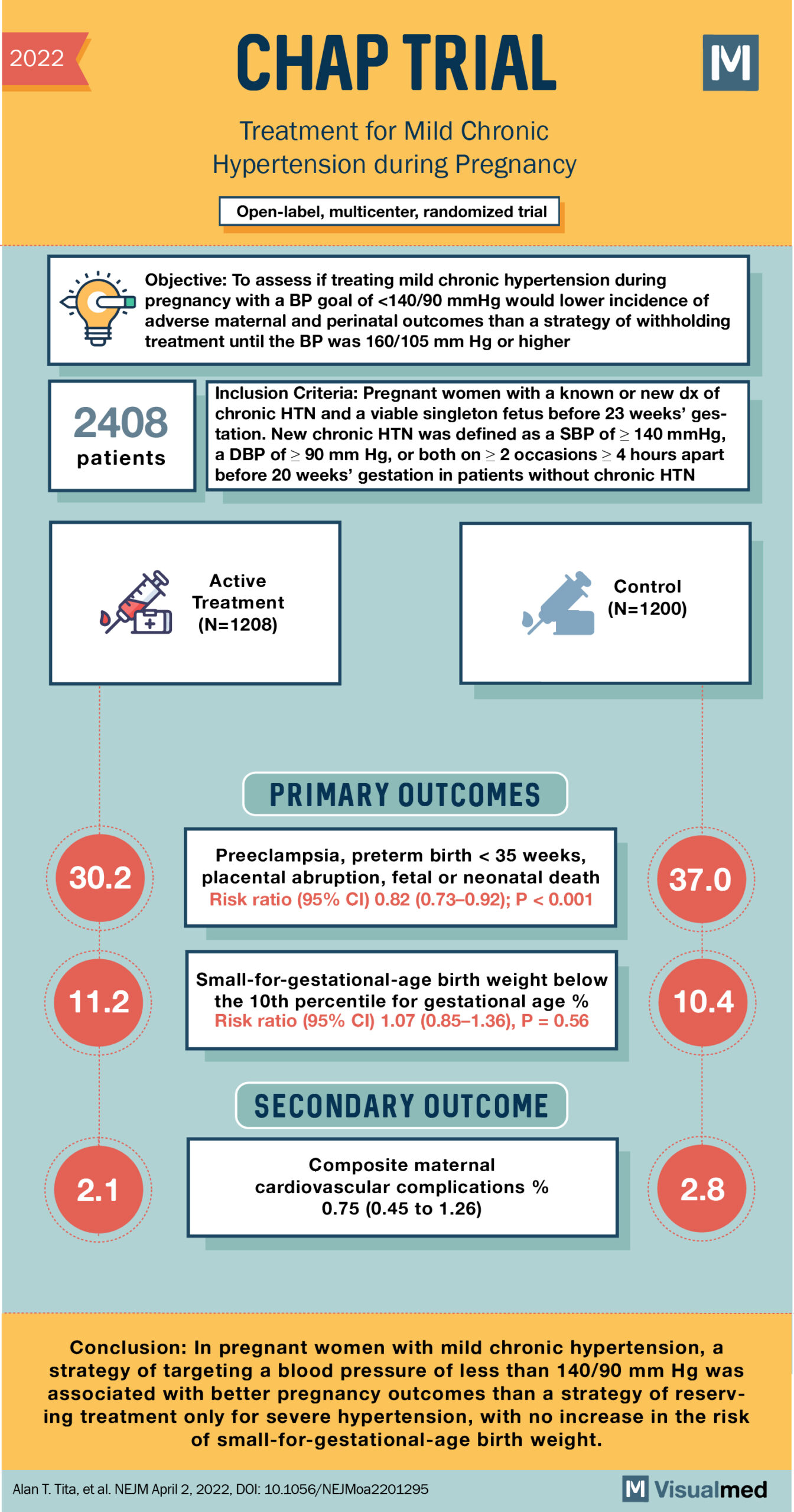
2022 CHAP TRIAL Treatment for Mild Chronic Hypertension during Pregnancy Open-label, multicenter, randomized trial Objective: To assess if treating mild chronic hypertension during pregnancy with a BP goal of <140/90 mmHg would lower incidence of adverse maternal and perinatal outcomes than a strategy of withholding treatment until the BP was 160/105 mm Hg or higher 2408 Inclusion Criteria: Pregnant women with a known or new dx of chronic HTN and a viable singleton fetus before 23 weeks’ gestation. New chronic HTN was defined as a SBP of > 140 mmHg, a DBP of > 90 mm Hg, or both on > 2 occasions > 4 hours apart before 20 weeks’ gestation in patients without chronic HTN patients Active Treatment (N=1208) Control (N=1200) PRIMARY OUTCOMES 30.2 Preeclampsia, preterm birth < 35 weeks, placental abruption, fetal or neonatal death Risk ratio (95% CI) 0.82 (0.73–0.92); P < 0.001 37.0 11.2 Small-for-gestational-age birth weight below the 10th percentile for gestational age % Risk ratio (95% CI) 1.07 (0.85-1.36), P = 0.56 10.4 SECONDARY OUTCOME 2.1 Composite maternal cardiovascular complications % 0.75 (0.45 to 1.26) 2.8 Conclusion: In pregnant women with mild chronic hypertension, a strategy of targeting a blood pressure of less than 140/90 mm Hg was associated with better pregnancy outcomes than a strategy of reserving treatment only for severe hypertension, with no increase in the risk of small-for-gestational-age birth weight. Alan T. Tita, et al. NEJM April 2, 2022, DOI: 10.1056/NEJMoa2201295