DAPA-MI Trial: Dapagliflozin in MI?
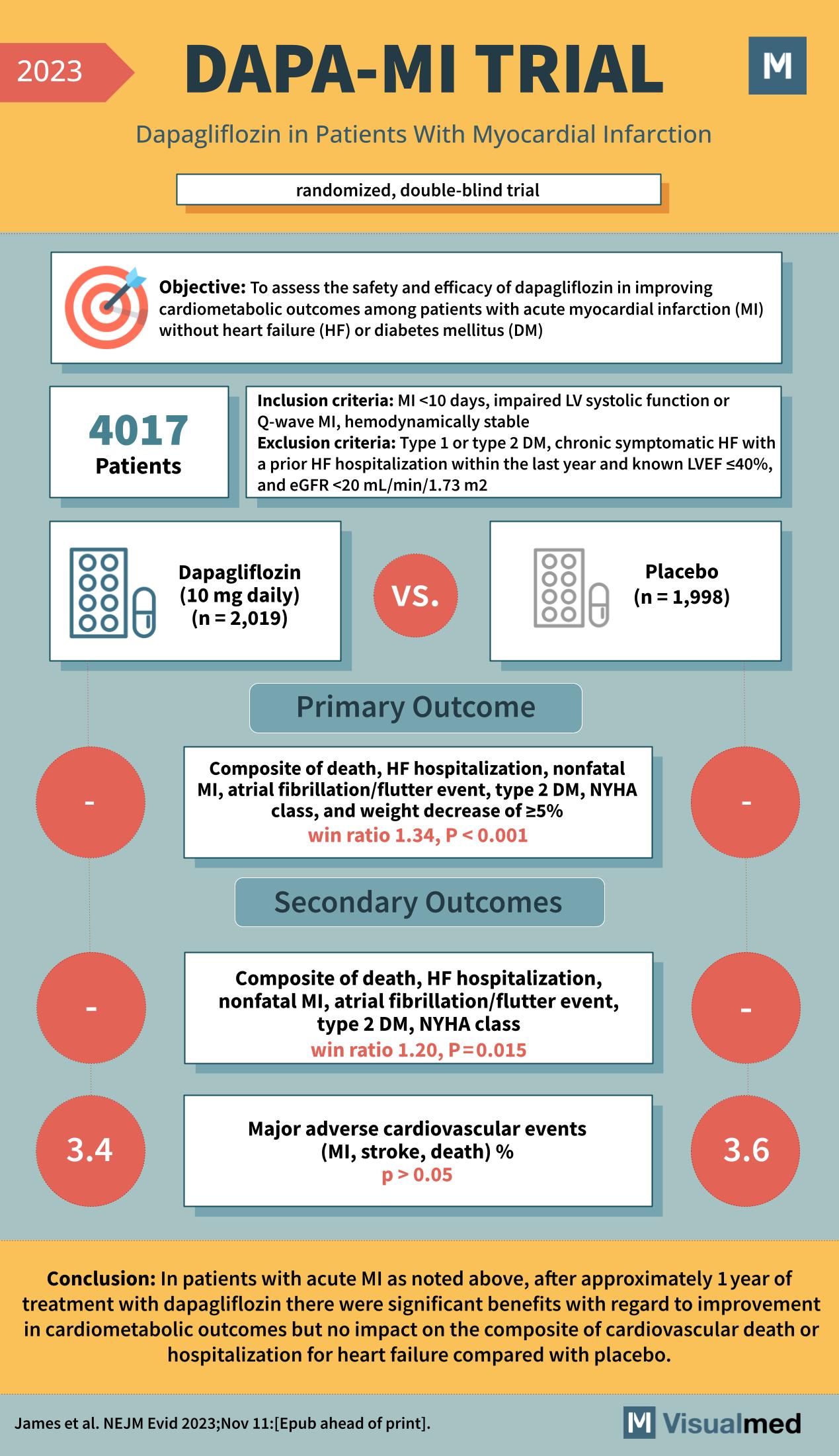
The DAPA-MI Trial, as reported in the New England Journal of Medicine in 2023, was a randomized, double-blind clinical trial designed to assess the safety and efficacy of dapagliflozin, a medication typically used to treat diabetes, in improving cardiometabolic outcomes for patients with acute myocardial infarction (MI) without heart failure (HF) or diabetes mellitus (DM).
The trial enrolled 4,017 patients who had experienced an MI less than 10 days prior, had impaired left ventricular systolic function or Q-wave MI, and were hemodynamically stable. The participants were randomized to receive either dapagliflozin (10 mg daily, n=2,019) or a placebo (n=1,998).
The primary outcome was a composite measure including death, HF hospitalization, nonfatal MI, atrial fibrillation/flutter event, type 2 DM, New York Heart Association (NYHA) class, and a weight decrease of ≥5%. The trial found that the primary outcome had a win ratio of 1.34, indicating a significant benefit with dapagliflozin treatment (P < 0.001).
Secondary outcomes included a composite of death, HF hospitalization, nonfatal MI, atrial fibrillation/flutter event, type 2 DM, NYHA class, with a win ratio of 1.20 (P=0.015), showing a benefit. However, there was no significant impact on the rate of major adverse cardiovascular events (MACE) such as MI, stroke, or death (3.4% in the dapagliflozin group vs. 3.6% in the placebo group, P > 0.05).
The conclusion of the DAPA-MI Trial was that in patients with acute MI, treatment with dapagliflozin for approximately one year led to significant benefits in terms of cardiometabolic outcomes but did not significantly impact the composite of cardiovascular death or hospitalization for heart failure when compared with placebo. This suggests that dapagliflozin may offer additional therapeutic benefits for patients post-MI beyond its known effects on glucose control.