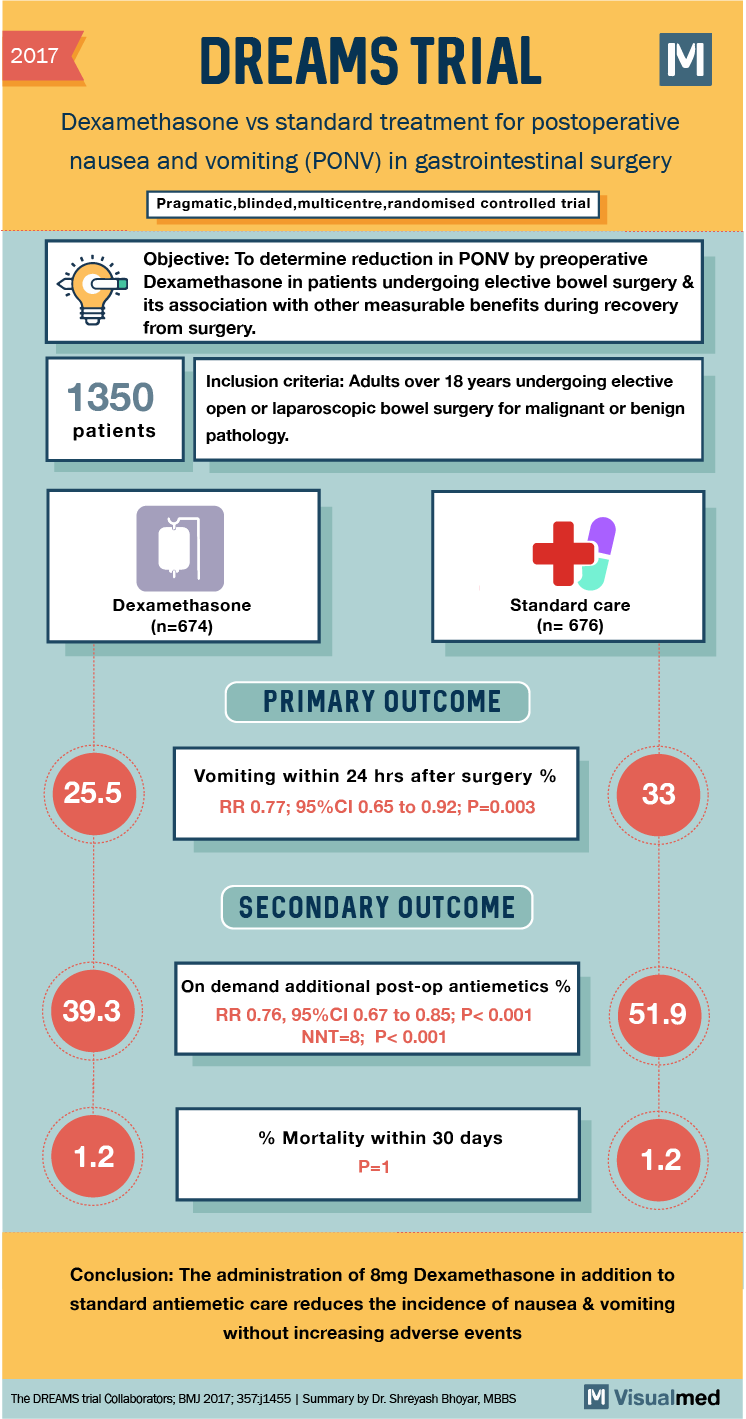
2017 DREAMS TRIAL Dexamethasone vs standard treatment for postoperative nausea and vomiting (PONV) in gastrointestinal surgery Pragmatic, blinded, multicentre,randomised controlled trial Objective: To determine reduction in PONV by preoperative Dexamethasone in patients undergoing elective bowel surgery & its association with other measurable benefits during recovery from surgery. 1350 patients Inclusion criteria: Adults over 18 years undergoing elective open or laparoscopic bowel surgery for malignant or benign pathology. Dexamethasone (n=674) Standard care (n= 676) PRIMARY OUTCOME 25.5 Vomiting within 24 hrs after surgery % RR 0.77; 95%CI 0.65 to 0.92; P=0.003 SECONDARY OUTCOME 39.3 On demand additional post-op antiemetics % RR 0.76, 95%CI 0.67 to 0.85; P<0.001 NNT=8; P<0.001 51.9 % Mortality within 30 days P=1 Conclusion: The administration of Smg Dexamethasone in addition to standard antiemetic care reduces the incidence of nausea & vomiting without increasing adverse events The DREAMS trial Collaborators, BMJ 2017, 357.j1455