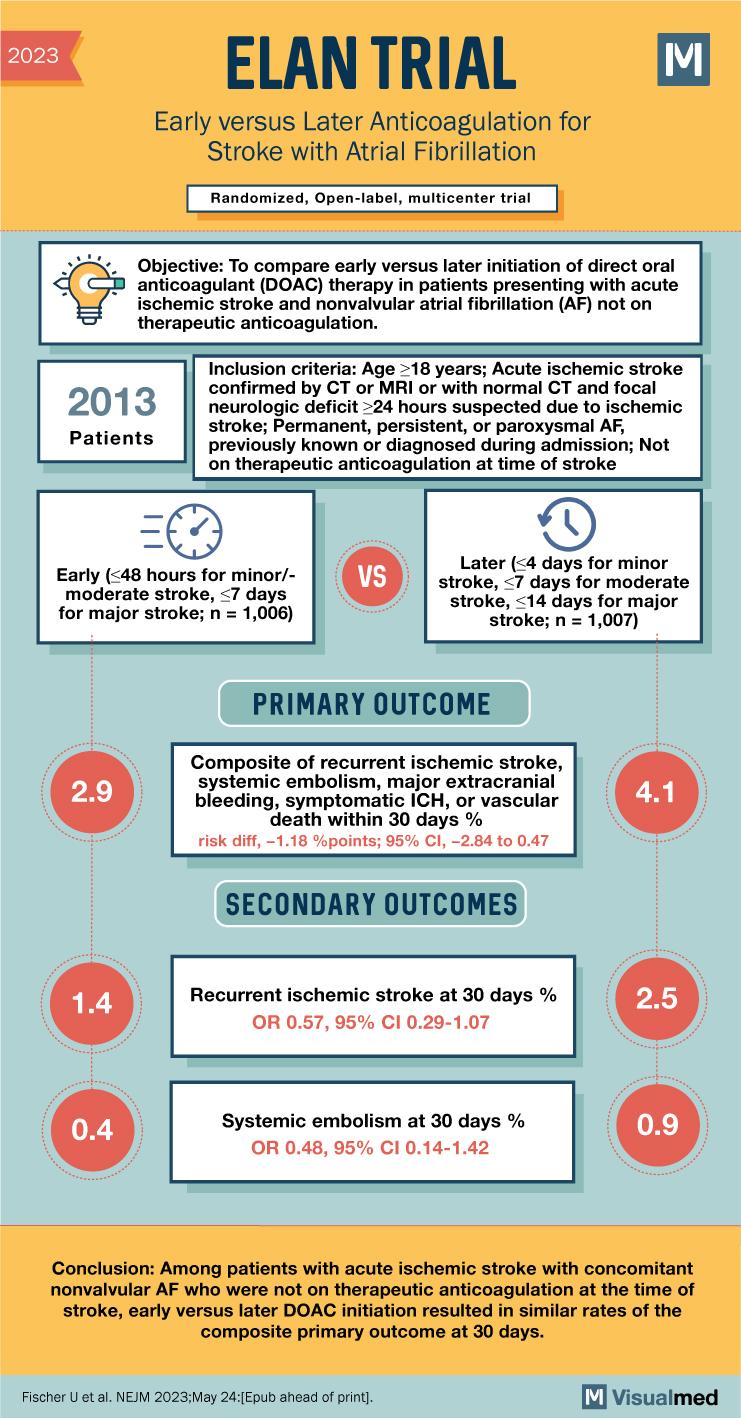
ELAN Trial Summary
Introduction:
The ELAN trial aimed to investigate the optimal timing of initiating direct oral anticoagulants (DOACs) in individuals with atrial fibrillation (AF) who had experienced an acute ischemic stroke. The study compared early anticoagulation (initiated within 48 hours or on day 6-7 after stroke) with later anticoagulation (initiated on day 3-4, day 6-7, or day 12-14 after stroke). The primary outcome was a composite measure of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Methods:
The trial was conducted at 103 sites across 15 countries. Key features of the study design included:
- Participants: Individuals with acute ischemic stroke confirmed by imaging or by persistent focal neurological deficit presumed to be of ischemic origin, along with a diagnosis of AF.
- Randomization: Participants were randomly assigned in a 1:1 ratio to either early anticoagulation or later anticoagulation.
- Primary Outcome: The primary outcome measure was the composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
- Secondary Outcomes: Secondary outcomes included individual components of the primary outcome at both 30 and 90 days.
Apologies for the oversight. Here are the inclusion and exclusion criteria for the ELAN trial:
Inclusion Criteria:
- Written informed consent according to country-specific details.
- Age: ≥18 years.
- Acute ischemic stroke confirmed by MRI or CT scan (tissue-based definition) or by sudden focal neurological deficit of presumed ischemic origin that persisted beyond 24 hours and a normal non-contrast CT scan.
- Permanent, persistent, or paroxysmal spontaneous atrial fibrillation (AF) previously known or diagnosed during the index hospitalization.
- Agreement of the treating physician to prescribe DOACs.
Exclusion Criteria:
- Atrial fibrillation due to reversible causes (e.g., thyrotoxicosis, pericarditis, recent surgery, myocardial infarct).
- Valvular disease requiring surgery.
- Mechanical heart valve(s).
- Moderate or severe mitral stenosis (other valvular diseases and biological valves are eligible).
- AF and conditions other than AF that require anticoagulation, including therapeutic dose of low-molecular-weight heparin or heparin (infratherapeutic anticoagulation at ischemic stroke onset is not an exclusion criterion).
- Contraindications to DOACs.
Please note that these criteria were used to select eligible participants for the trial.
Results:
A total of 2,013 participants were enrolled in the trial, with 1,006 assigned to early anticoagulation and 1,007 to later anticoagulation. The majority of participants had minor or moderate strokes. The primary outcome occurred in 2.9% of the early-treatment group and 4.1% of the later-treatment group by 30 days. Recurrent ischemic stroke was observed in 1.4% of the early-treatment group and 2.5% of the later-treatment group by 30 days, and in 1.9% and 3.1% respectively by 90 days. Symptomatic intracranial hemorrhage occurred in 0.2% of participants in both groups by 30 days.
Conclusion:
The ELAN trial found that the timing of initiating DOACs in patients with acute ischemic stroke and AF did not significantly impact the incidence of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death at 30 days. The results indicated a range of the primary outcome estimate that was 2.8 percentage points lower to 0.5 percentage points higher for early initiation compared to later initiation of DOACs, based on the 95% confidence interval. These findings suggest that both early and later initiation of DOACs may be reasonable approaches in this patient population, with no significant differences observed between the two groups.