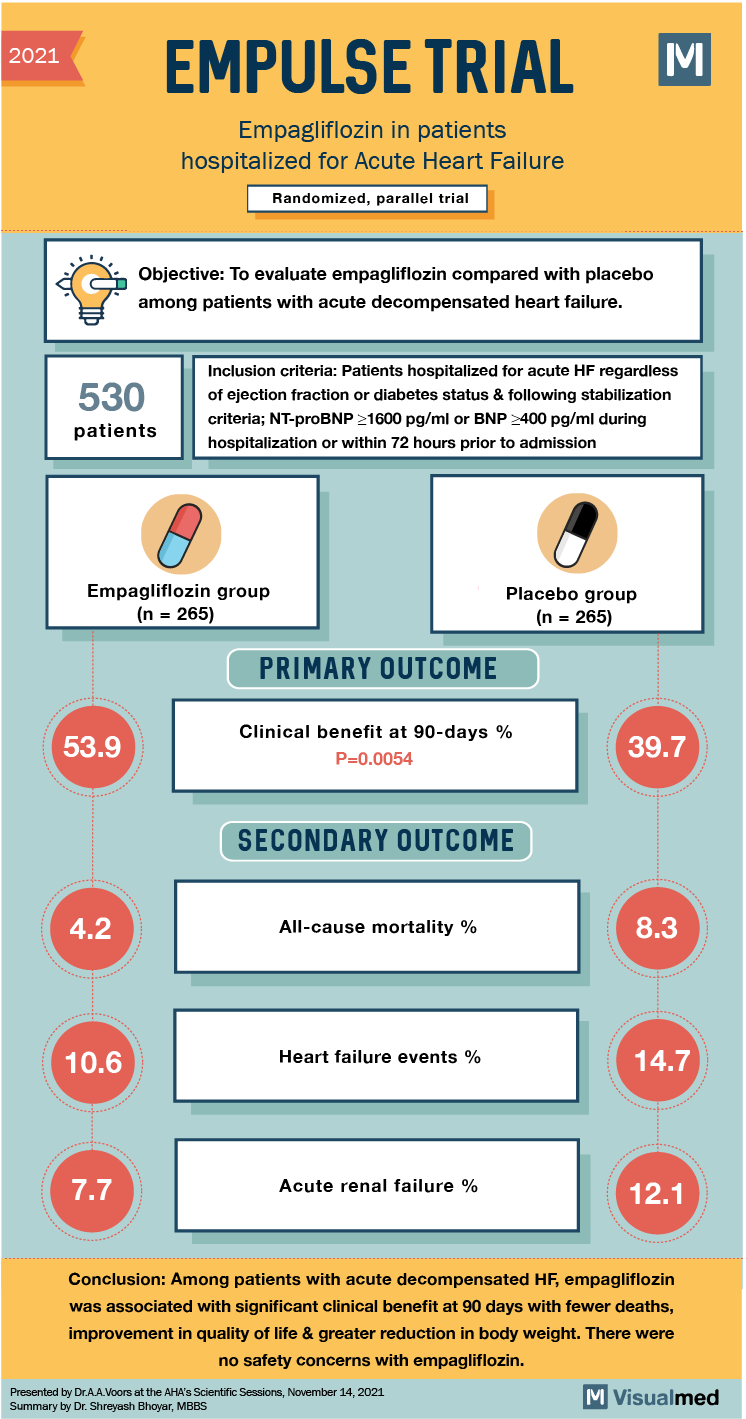
2021 EMPULSE TRIAL Empagliflozin in patients hospitalized for Acute Heart Failure Randomized, parallel trial Objective: To evaluate empagliflozin compared with placebo among patients with acute decompensated heart failure. 530 patients Inclusion criteria: Patients hospitalized for acute HF regardless of ejection fraction or diabetes status & following stabilization criteria; NT-proBNP >1600 pg/ml or BNP 2400 pg/ml during hospitalization or within 72 hours prior to admission Empagliflozin group (n = 265) Placebo group (n = 265) PRIMARY OUTCOME 53.9 Clinical benefit at 90-days % P=0.0054 39.7 SECONDARY OUTCOME 4 All-cause mortality % 8.3 10.6 Heart failure events % 14.7 7.7 Acute renal failure % 12.1 Conclusion: Among patients with acute decompensated HF, empagliflozin was associated with significant clinical benefit at 90 days with fewer deaths, improvement in quality of life & greater reduction in body weight. There were no safety concerns with empagliflozin. Presented by Dr.A.A.Voors at the AHA’s Scientific Sessions, November 14, 2021