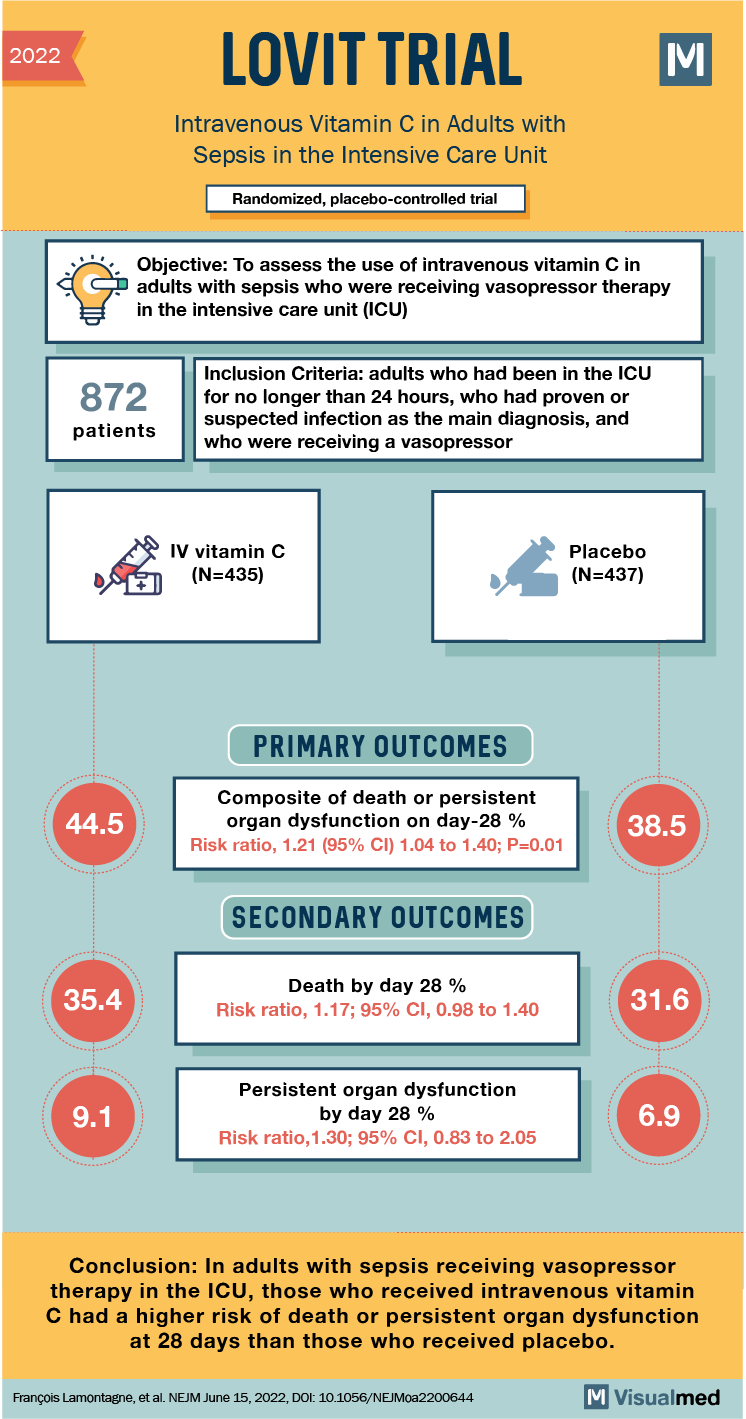
2022 LOVIT TRIAL Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit Randomized, placebo-controlled trial Objective: To assess the use of intravenous vitamin C in a dults with sepsis who were receiving vasopressor therapy in the intensive care unit (ICU) 872 patients 872 Inclusion Criteria: adults who had been in the ICU for no longer than 24 hours, who had proven or suspected infection as the main diagnosis, and who were receiving a vasopressor ore to IV vitamin C DH (N=435) Placebo (N=437) PRIMARY OUTCOMES 44.5 Composite of death or persistent organ dysfunction on day-28 % Risk ratio, 1.21 (95% CI) 1.04 to 1.40; P=0.01 38.5 SECONDARY OUTCOMES 35.4 Death by day 28 % Risk ratio, 1.17; 95% CI, 0.98 to 1.40 31.6 9.1 Persistent organ dysfunction by day 28 % Risk ratio, 1.30; 95% CI, 0.83 to 2.05 6.9 Conclusion: In adults with sepsis receiving vasopressor therapy in the ICU, those who received intravenous vitamin C had a higher risk of death or persistent organ dysfunction at 28 days than those who received placebo. François Lamontagne, et al. NEJM June 15, 2022, DOI: 10.1056/NEJMoa2200644 M Visualmed