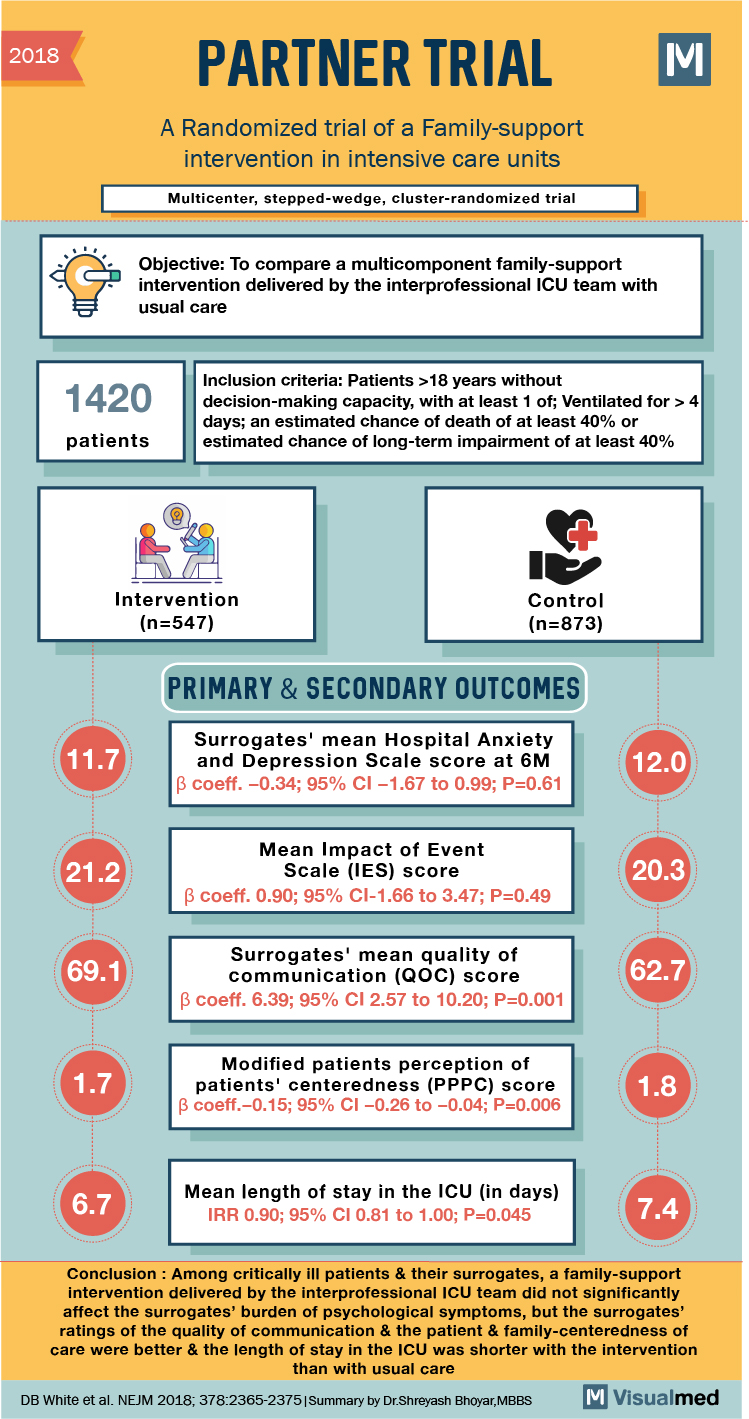
2018 PARTNER TRIAL A Randomized trial of a Family-support intervention in intensive care units Multicenter, stepped-wedge, cluster-randomized trial Objective: To compare a multicomponent family-support intervention delivered by the interprofessional ICU team with usual care 1420 Inclusion criteria: Patients >18 years without decision-making capacity, with at least 1 of; Ventilated for > 4 days; an estimated chance of death of at least 40% or estimated chance of long-term impairment of at least 40% patients Intervention (n=547) Control (n=873) PRIMARY & SECONDARY OUTCOMES 11.7 Surrogates’ mean Hospital Anxiety and Depression Scale score at 6M B coeff. -0.34; 95% CI -1.67 to 0.99; P=0.61 12.0 21.2 Mean Impact of Event Scale (IES) score B coeff. 0.90; 95% CI-1.66 to 3.47; P=0.49 20.3 69.1 Surrogates’ mean quality of communication (QOC) score B coeff. 6.39; 95% CI 2.57 to 10.20; P=0.001 62.7 1.7 Modified patients perception of patients’ centeredness (PPPC) score B coeff.-0.15; 95% CI -0.26 to -0.04; P=0.006 1.8 6.7 Mean length of stay in the ICU (in days) IRR 0.90; 95% CI 0.81 to 1.00; P=0.045 7.4 Conclusion : Among critically ill patients & their surrogates, a family-support intervention delivered by the interprofessional ICU team did not significantly affect the surrogates’ burden of psychological symptoms, but the surrogates’ ratings of the quality of communication & the patient & family-centeredness of care were better & the length of stay in the ICU was shorter with the intervention than with usual care DB White et al. NEJM 2018; 378:2365-2375