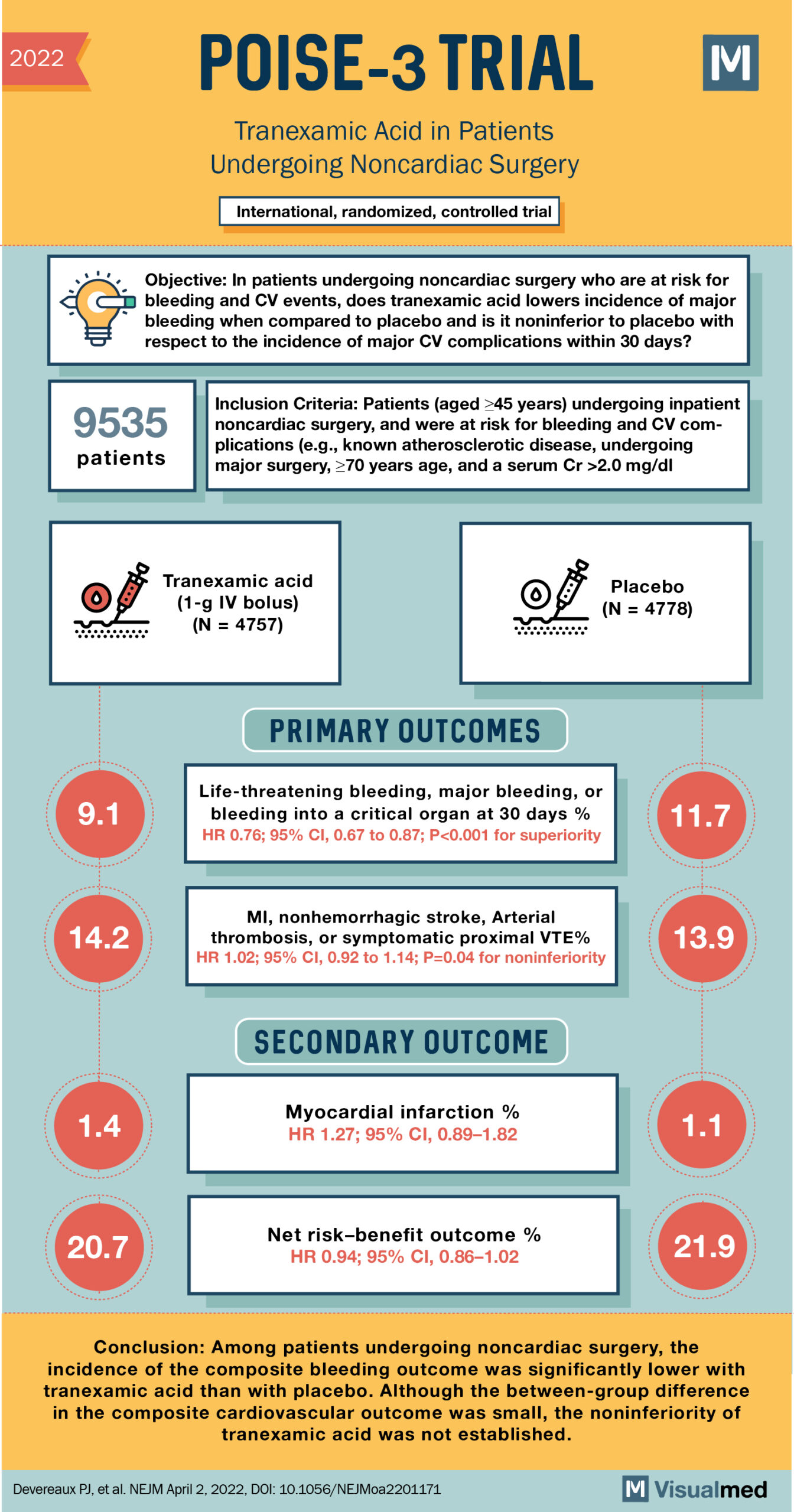
2022 POISE-3 TRIAL МІ Tranexamic Acid in Patients Undergoing Noncardiac Surgery International, randomized, controlled trial Objective: In patients undergoing noncardiac surgery who are at risk for bleeding and CV events, does tranexamic acid lowers incidence of major bleeding when compared to placebo and is it noninferior to placebo with respect to the incidence of major CV complications within 30 days? 9535 Inclusion Criteria: Patients (aged >45 years) undergoing inpatient noncardiac surgery, and were at risk for bleeding and CV complications (e.g., known atherosclerotic disease, undergoing major surgery, >70 years age, and a serum Cr >2.0 mg/dl patients Tranexamic acid (1-g IV bolus) (N = 4757) Placebo (N = 4778) PRIMARY OUTCOMES 9.1 Life-threatening bleeding, major bleeding, or bleeding into a critical organ at 30 days % HR 0.76; 95% CI, 0.67 to 0.87; P<0.001 for superiority 11.7 14.2 MI, nonhemorrhagic stroke, Arterial thrombosis, or symptomatic proximal VTE% HR 1.02; 95% CI, 0.92 to 1.14; P=0.04 for noninferiority 13.9 SECONDARY OUTCOME 1.4 Myocardial infarction % HR 1.27; 95% CI, 0.89–1.82 20.7 Net risk-benefit outcome % HR 0.94; 95% CI, 0.86–1.02 21.9 Conclusion: Among patients undergoing noncardiac surgery, the incidence of the composite bleeding outcome was significantly lower with tranexamic acid than with placebo. Although the between-group difference in the composite cardiovascular outcome was small, the noninferiority of tranexamic acid was not established. Devereaux PJ, et al. NEJM April 2, 2022, DOI: 10.1056/NEJMoa2201171