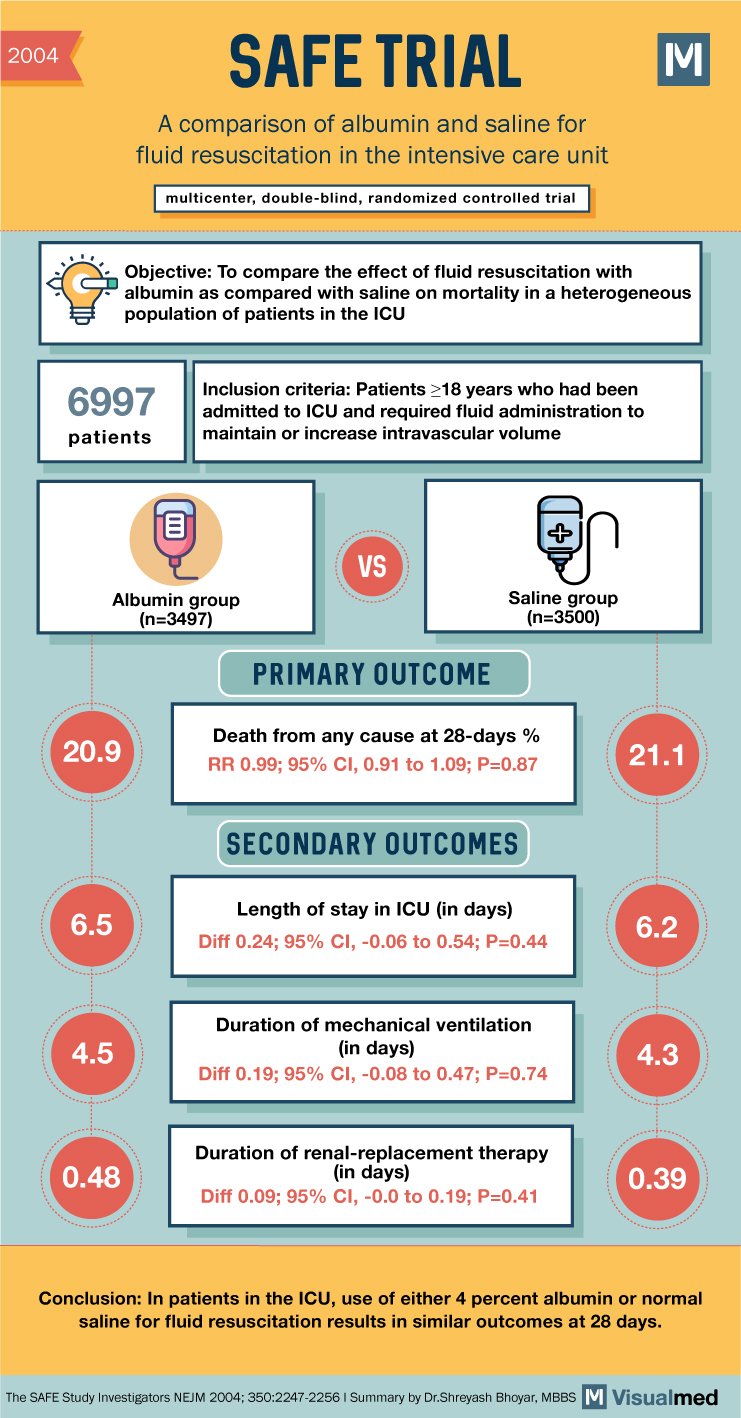
2004 SAFE TRIAL A comparison of albumin and saline for fluid resuscitation in the intensive care unit multicenter, double-blind, randomized controlled trial M Objective: To compare the effect of fluid resuscitation with albumin as compared with saline on mortality in a heterogeneous population of patients in the ICU 6997 patients 目 Inclusion criteria: Patients ≥18 years who had been admitted to ICU and required fluid administration to maintain or increase intravascular volume VS Albumin group (n=3497) Saline group (n=3500) 20.9 PRIMARY OUTCOME Death from any cause at 28-days% RR 0.99; 95% CI, 0.91 to 1.09; P=0.87 SECONDARY OUTCOMES 21.1 Length of stay in ICU (in days) 6.5 6.2 Diff 0.24; 95% CI, -0.06 to 0.54; P=0.44 4.5 Duration of mechanical ventilation (in days) 4.3 Diff 0.19; 95% CI, -0.08 to 0.47; P=0.74 Duration of renal-replacement therapy 0.48 (in days) 0.39 Diff 0.09; 95% CI, -0.0 to 0.19; P=0.41 Conclusion: In patients in the ICU, use of either 4 percent albumin or normal saline for fluid resuscitation results in similar outcomes at 28 days. The SAFE Study Investigators NEJM 2004; 350:2247-2256