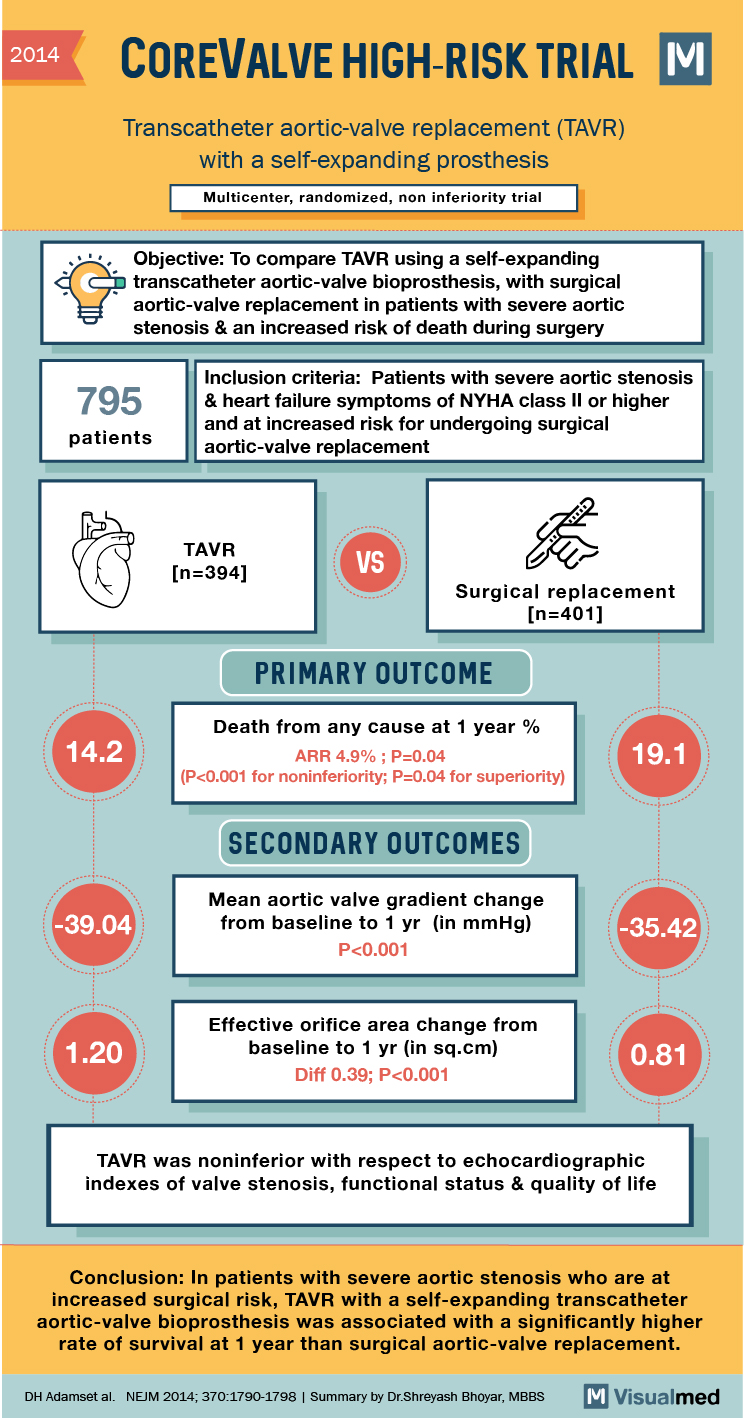
2014 COREVALVE HIGH-RISK TRIAL Transcatheter aortic-valve replacement (TAVR) with a self-expanding prosthesis Multicenter, randomized, non inferiority trial “‘Objective: To compare TAVR using a self-expanding a transcatheter aortic-valve bioprosthesis, with surgical aortic-valve replacement in patients with severe aortic stenosis & an increased risk of death during surgery 795 Inclusion criteria: Patients with severe aortic stenosis & heart failure symptoms of NYHA class Il or higher and at increased risk for undergoing surgical aortic-valve replacement patients TAVR [n=394] VS Surgical replacement (n=401] PRIMARY OUTCOME 14.2 Death from any cause at 1 year % ARR 4.9%; P=0.04 (P<0.001 for noninferiority; P=0.04 for superiority) 19.1 SECONDARY OUTCOMES -39.04 Mean aortic valve gradient change from baseline to 1 yr (in mmHg) P<0.001 -35.42 1.20 Effective orifice area change from baseline to 1 yr (in sq.cm) Diff 0.39; P<0.001 0.81 TAVR was noninferior with respect to echocardiographic indexes of valve stenosis, functional status & quality of life Conclusion: In patients with severe aortic stenosis who are at increased surgical risk, TAVR with a self-expanding transcatheter aortic-valve bioprosthesis was associated with a significantly higher rate of survival at 1 year than surgical aortic-valve replacement. DH Adamset al. NEJM 2014; 370:1790-1798