Heart failure with preserved ejection fraction (HFpEF) is a complex and increasingly prevalent form of heart failure, accounting for nearly half of all heart failure cases. Despite its significance, HFpEF remains a challenging condition to manage and treat, as effective therapies have been limited. In this article, we delve into the landmark clinical trials that have shaped our understanding of HFpEF, its pathophysiology, and the development of targeted treatments.
CHARM-Preserved (2003)
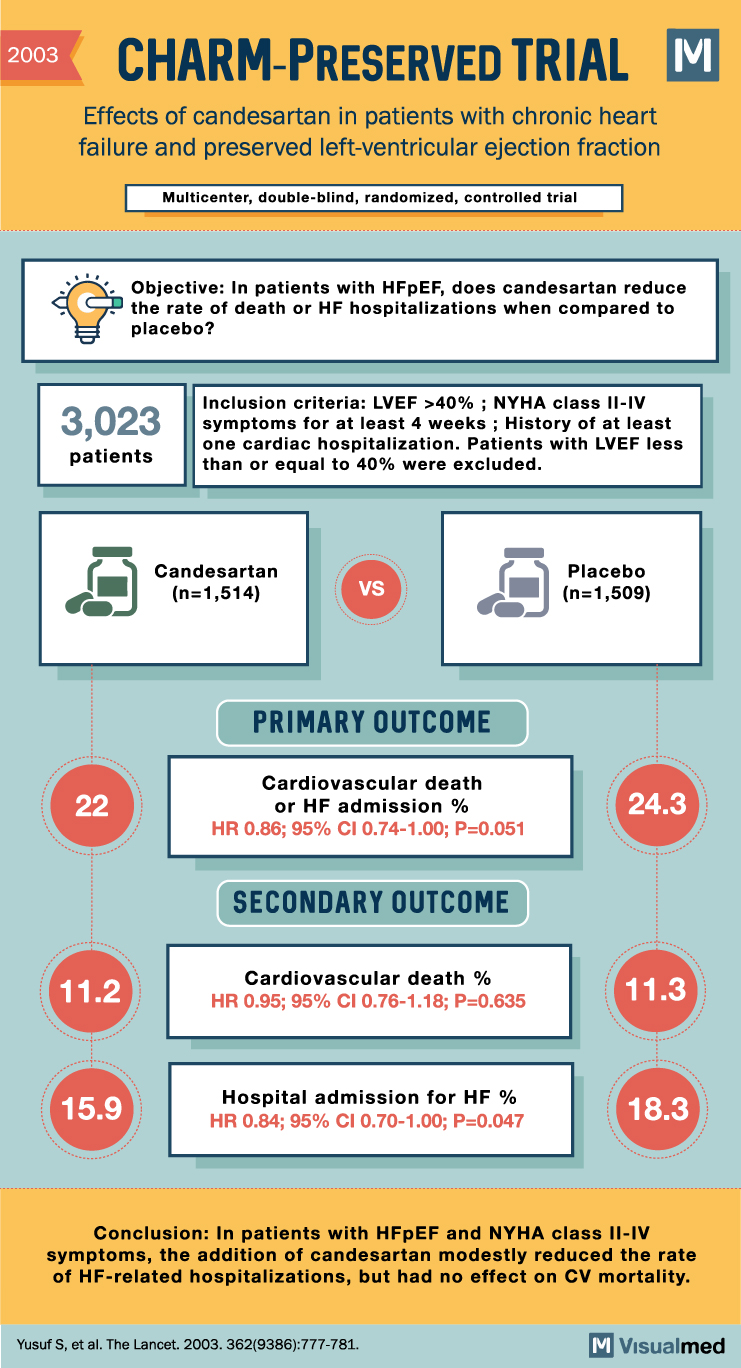
The Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM)-Preserved trial was one of the earliest large-scale clinical trials focused on HFpEF. This study assessed the effect of the angiotensin receptor blocker (ARB) candesartan on cardiovascular death and hospitalization due to heart failure in patients with HFpEF.
Results from CHARM-Preserved demonstrated that candesartan modestly reduced heart failure hospitalizations but had no significant impact on cardiovascular mortality. These findings highlighted the need for further research and development of targeted therapies for HFpEF.
I-PRESERVE (2008)
The Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) aimed to evaluate the efficacy of another ARB, irbesartan, in reducing morbidity and mortality in HFpEF patients. Unfortunately, I-PRESERVE results showed that irbesartan did not significantly reduce the composite outcome of death from any cause or hospitalization due to cardiovascular reasons.
These negative findings raised questions about the efficacy of ARBs in HFpEF and prompted the search for more effective treatment options.
TOPCAT (2014)
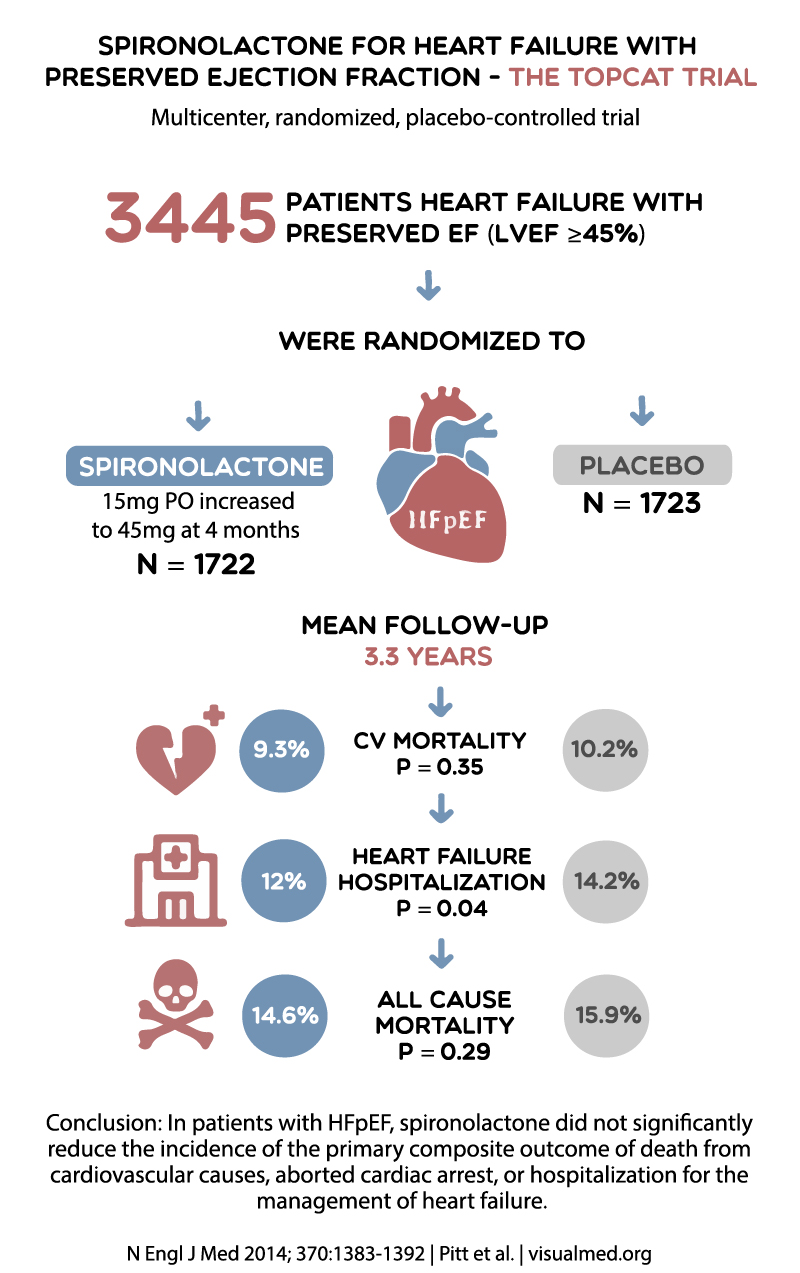
The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial investigated the mineralocorticoid receptor antagonist spironolactone’s effectiveness in HFpEF patients. The primary composite outcome included death from cardiovascular causes, aborted cardiac arrest, or hospitalization for heart failure.
TOPCAT’s results were mixed, as spironolactone failed to significantly reduce the primary composite outcome in the overall study population. However, a post-hoc analysis suggested a possible benefit in specific patient subgroups, such as those with elevated natriuretic peptide levels. This trial emphasized the need for better patient selection and personalized therapeutic approaches in HFpEF.
PARAMOUNT (2012)
The Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) trial was a groundbreaking study that introduced a new class of medications, angiotensin receptor-neprilysin inhibitors (ARNIs). The trial assessed the efficacy and safety of the ARNI sacubitril/valsartan (LCZ696) compared to the ARB valsartan in HFpEF patients.
The PARAMOUNT trial demonstrated that sacubitril/valsartan led to a more significant reduction in the biomarker N-terminal pro-B-type natriuretic peptide (NT-proBNP) than valsartan alone. Additionally, sacubitril/valsartan was associated with a reduction in left atrial size, suggesting potential benefits in treating HFpEF. These promising results led to further investigation of ARNIs in HFpEF patients.
PARAGON-HF (2019)
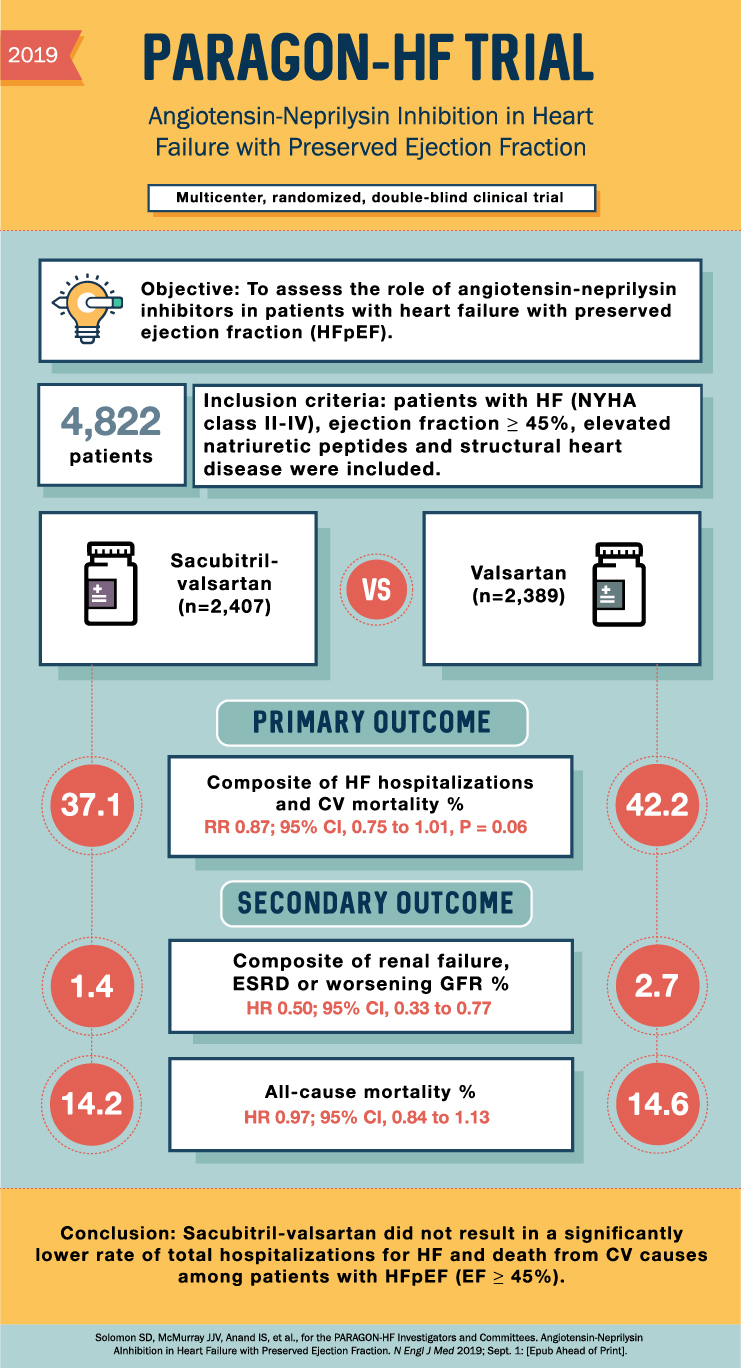
Following the encouraging findings from PARAMOUNT, the Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF (PARAGON-HF) trial was designed to evaluate the effect of sacubitril/valsartan on morbidity and mortality in a larger HFpEF population. The primary composite outcome included total hospitalizations for heart failure and death from cardiovascular causes.
Although PARAGON-HF did not demonstrate a significant reduction in the primary composite outcome for the overall population, a subgroup analysis revealed a potential benefit in specific patient populations, such as women and those with a left ventricular ejection fraction closer to 45%. This trial highlighted the importance of further refining patient selection and targeting specific subgroups for optimized HFpEF treatment.
EMPEROR-Preserved (2021)
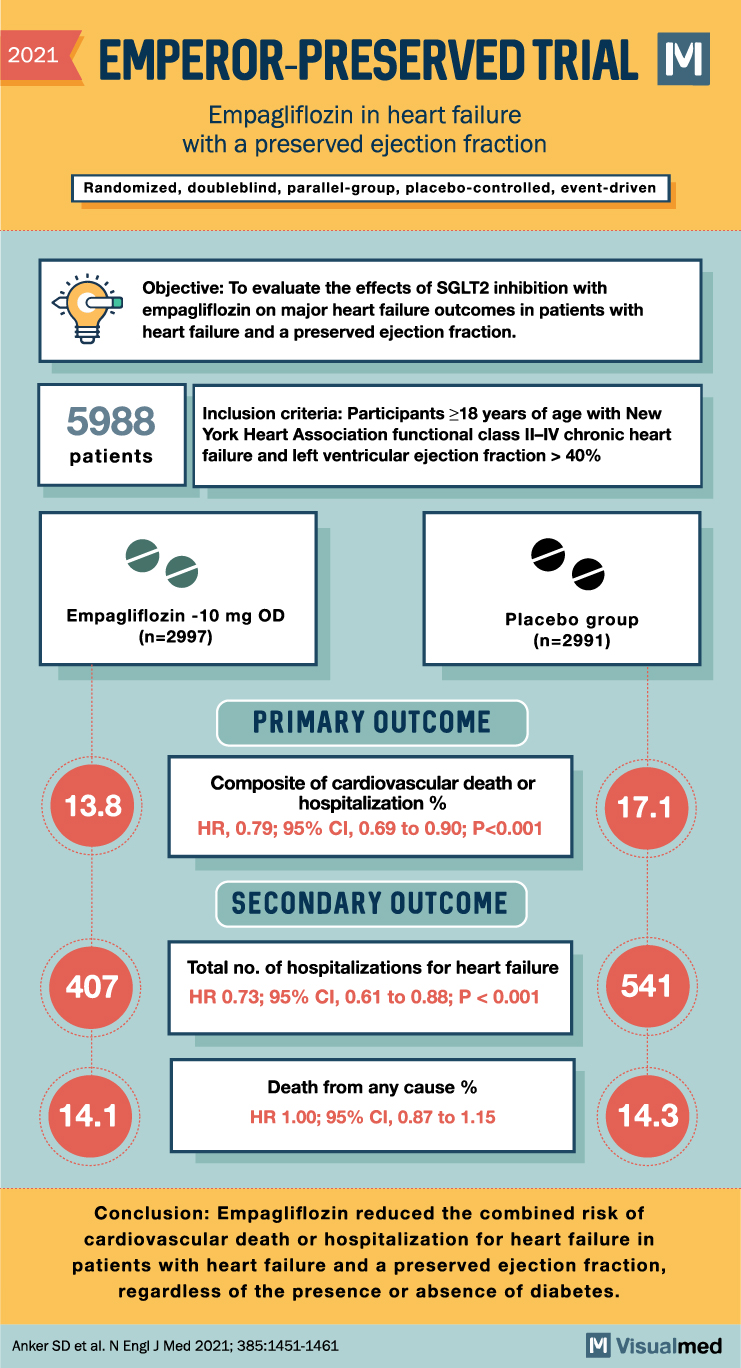
The EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved) trial was a landmark study that investigated the efficacy of the sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin in reducing cardiovascular death and heart failure hospitalizations in HFpEF patients.
The results of EMPEROR-Preserved showed a significant reduction in the combined risk of cardiovascular death and heart failure hospitalizations with empagliflozin compared to placebo. This trial marked a significant breakthrough in HFpEF treatment, as it was the first to demonstrate a clear benefit with a novel therapeutic agent in this patient population.
Conclusion
The journey to finding effective treatments for HFpEF has been a long and challenging one, with numerous clinical trials conducted over the past two decades. While some trials, such as CHARM-Preserved, I-PRESERVE, and TOPCAT, yielded mixed or disappointing results, others like PARAMOUNT, PARAGON-HF, and EMPEROR-Preserved have paved the way for improved understanding and targeted treatment approaches.
The success of the EMPEROR-Preserved trial with empagliflozin has generated renewed optimism and interest in HFpEF research. It underscores the importance of continued investigation into novel therapeutic strategies and personalized treatment approaches to improve outcomes for patients with heart failure with preserved ejection fraction.
As our understanding of HFpEF continues to evolve, future clinical trials will undoubtedly refine our approach to managing this complex condition, leading to a better quality of life and outcomes for affected individuals.
References:
- Yusuf, S., Pfeffer, M. A., Swedberg, K., Granger, C. B., Held, P., McMurray, J. J., … & Ostergren, J. (2003). Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet, 362(9386), 777-781.
- Massie, B. M., Carson, P. E., McMurray, J. J., Komajda, M., McKelvie, R., Zile, M. R., … & Ptaszynska, A. (2008). Irbesartan in patients with heart failure and preserved ejection fraction. New England Journal of Medicine, 359(23), 2456-2467.
- Pitt, B., Pfeffer, M. A., Assmann, S. F., Boineau, R., Anand, I. S., Claggett, B., … & Solomon, S. D. (2014). Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine, 370(15), 1383-1392.
- Solomon, S. D., Zile, M., Pieske, B., Voors, A., Shah, A., Kraigher-Krainer, E., … & Packer, M. (2012). The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. The Lancet, 380(9851), 1387-1395.
- Solomon, S. D., McMurray, J. J., Anand, I. S., Ge, J., Lam, C. S., Maggioni, A. P., … & Lewis, E. F. (2019). Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. New England Journal of Medicine, 381(17), 1609-1620.
- Anker, S. D., Butler, J., Filippatos, G., Ferreira, J. P., Bocchi, E., Böhm, M., … & Pocock, S. J. (2021). Empagliflozin in heart failure with a preserved ejection fraction. New England Journal of Medicine, 385(16), 1451-1461.