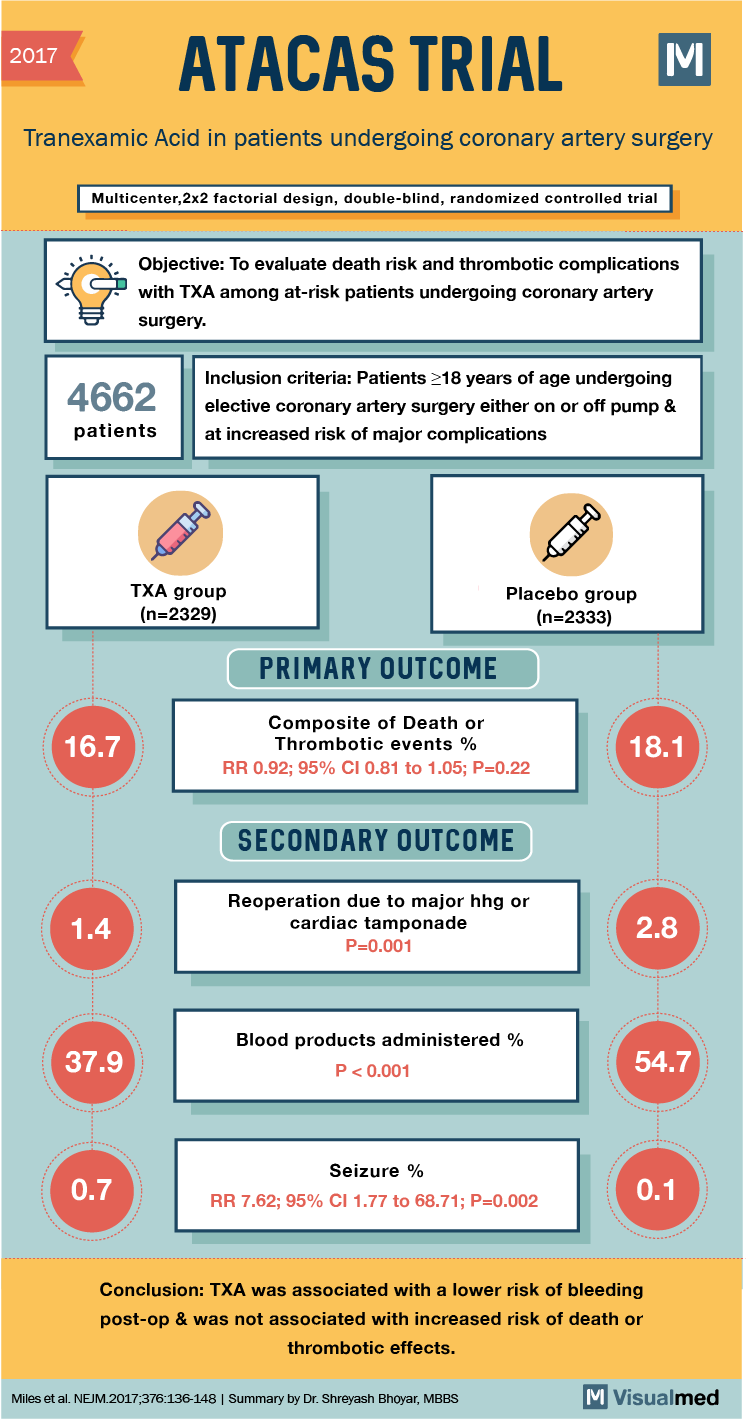
2017 ATACAS TRIAL: Tranexamic Acid in patients undergoing coronary artery surgery Multicenter,2×2 factorial design, double-blind, randomized controlled trial Objective: To evaluate death risk and thrombotic complications with TXA among at-risk patients undergoing coronary artery surgery. 4662 Inclusion criteria: Patients >18 years of age undergoing elective coronary artery surgery either on or off pump & at increased risk of major complications patients OMEO TXA group (n=2329) Placebo group (n=2333) PRIMARY OUTCOME 16.7 Composite of Death or Thrombotic events % RR 0.92; 95% CI 0.81 to 1.05; P=0.22 18.1 SECONDARY OUTCOME 1.4 Reoperation due to major hhg or cardiac tamponade P=0.001 2.8 37.9 Blood products administered % P<0.001 54.7 0.7 Seizure % RR 7.62; 95% CI 1.77 to 68.71; P=0.002 0.1 Conclusion: TXA was associated with a lower risk of bleeding post-op & was not associated with increased risk of death or thrombotic effects. Miles et al. NEJM.2017,376:136-148