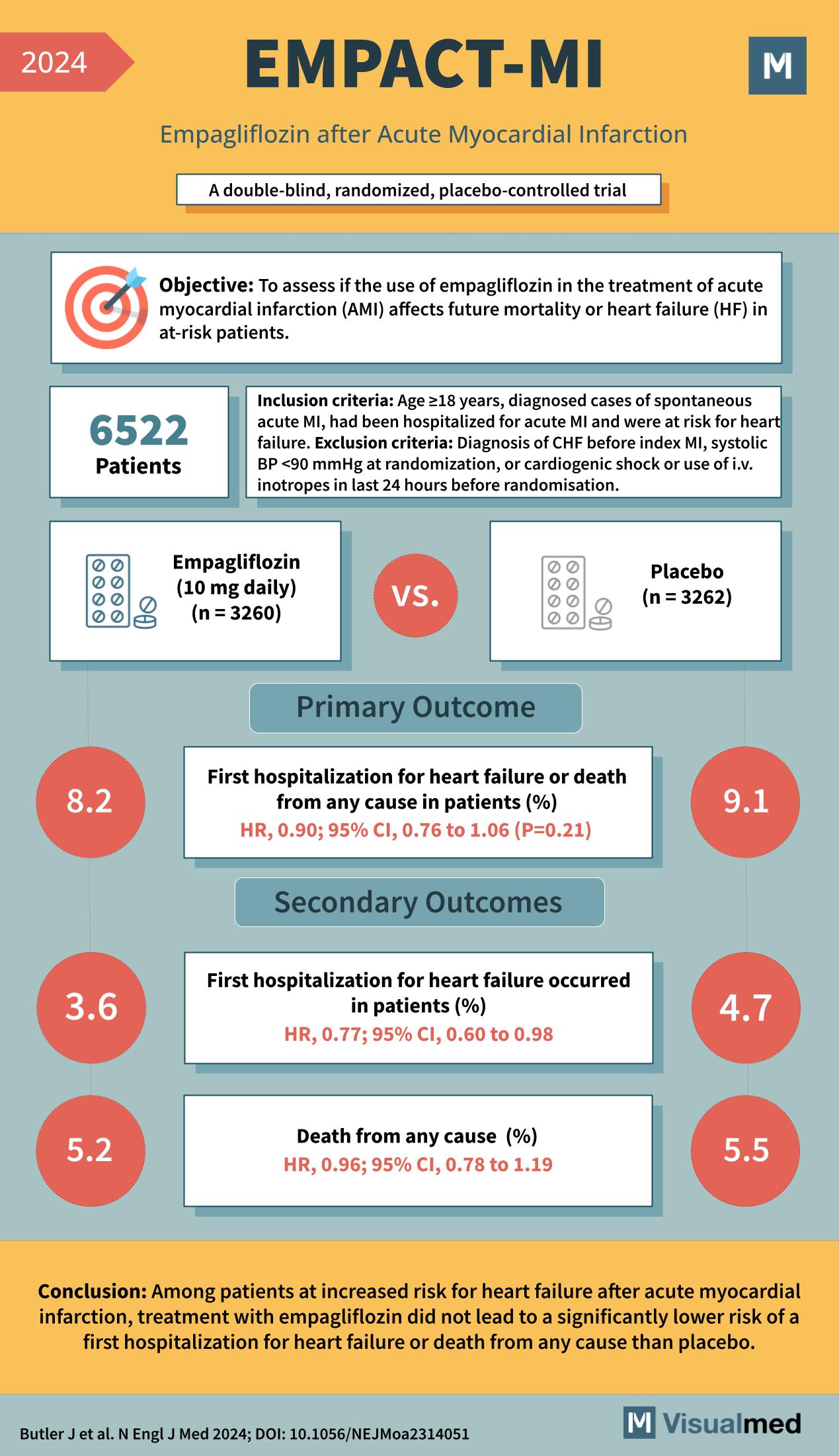
Year: 2024 Title: EMPACT-MI Subtitle: Empagliflozin after Acute Myocardial Infarction Type of Trial: A double-blind, randomized, placebo-controlled trial
Objective: To assess if the use of empagliflozin in the treatment of acute myocardial infarction (AMI) affects future mortality or heart failure (HF) in at-risk patients.
Patients: 6522
Inclusion Criteria:
- Age ≥18 years
- Diagnosed cases of spontaneous acute MI
- Had been hospitalized for acute MI and were at risk for heart failure.
Exclusion Criteria:
- Diagnosis of CHF before index MI
- Systolic BP <90 mmHg at randomization
- Cardiogenic shock or use of i.v. inotropes in the last 24 hours before randomization
Groups:
- Empagliflozin (10 mg daily) group (n = 3260)
- Placebo group (n = 3262)
Primary Outcome:
- First hospitalization for heart failure or death from any cause in patients (%)
- HR 0.90; 95% CI, 0.76 to 1.06 (P=0.21)
- Empagliflozin group: 8.2%
- Placebo group: 9.1%
Secondary Outcomes:
- First hospitalization for heart failure occurred in patients (%)
- HR 0.77; 95% CI, 0.60 to 0.98
- Empagliflozin group: 3.6%
- Placebo group: 4.7%
- Death from any cause (%)
- HR 0.96; 95% CI, 0.78 to 1.19
- Empagliflozin group: 5.2%
- Placebo group: 5.5%
Conclusion: Among patients at increased risk for heart failure after acute myocardial infarction, treatment with empagliflozin did not lead to a significantly lower risk of a first hospitalization for heart failure or death from any cause than placebo.
Reference: Butler J et al. N Engl J Med 2024; DOI: 10.1056/NEJMoa2314051