Explore the pivotal clinical trials that have shaped our understanding of the role of steroids in the management of septic shock, unveiling the complexities and nuances of this controversial topic.
Introduction
Septic shock is a life-threatening condition characterized by a dysregulated host response to infection, leading to circulatory and metabolic abnormalities. The use of steroids in septic shock management has long been a topic of debate among clinicians and researchers. Various clinical trials have been conducted to evaluate the efficacy and safety of steroid administration in septic shock patients. In this article, we will review the landmark clinical trials that have significantly impacted our understanding and approach to steroids use in septic shock management.
1. Reversal of late septic shock with supraphysiologic doses of hydrocortisone (1998)
The trial was a randomized, double-blind, placebo-controlled trial that assessed the effect of low-dose corticosteroids (hydrocortisone) in patients with septic shock who had a poor response to fluid resuscitation and vasopressor therapy. The primary outcome was 28-day all-cause mortality.
Results from the RALES trial showed that low-dose hydrocortisone significantly reduced 28-day mortality in septic shock patients with poor response to initial resuscitative measures. This landmark trial provided the foundation for the use of low-dose corticosteroids in septic shock management.
2. Corticosteroid Therapy of Septic Shock (CORTICUS) (2008)
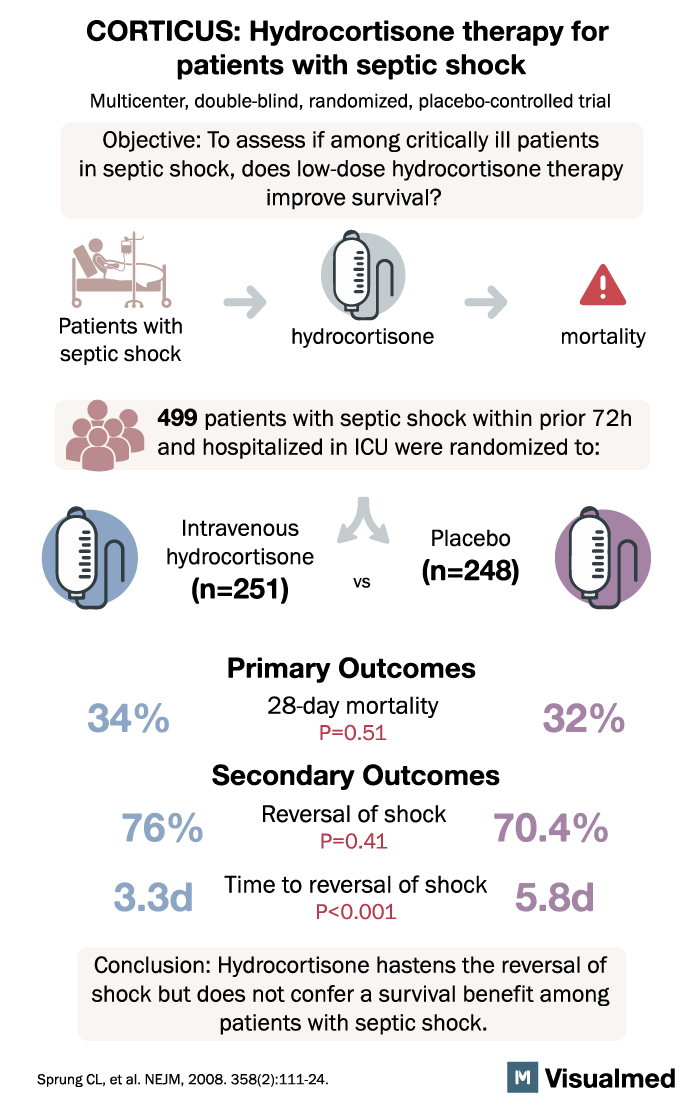
The CORTICUS trial aimed to evaluate the efficacy of hydrocortisone in septic shock patients, particularly those with relative adrenal insufficiency. The primary outcome was 28-day all-cause mortality.
Contrary to the 1998 trial, the CORTICUS trial found no significant difference in 28-day mortality between the hydrocortisone group and the placebo group. This study raised questions about the universal application of steroids in septic shock and highlighted the need for more research to identify specific patient populations that may benefit from steroid therapy.
3. The HYPRESS Trial (2016)
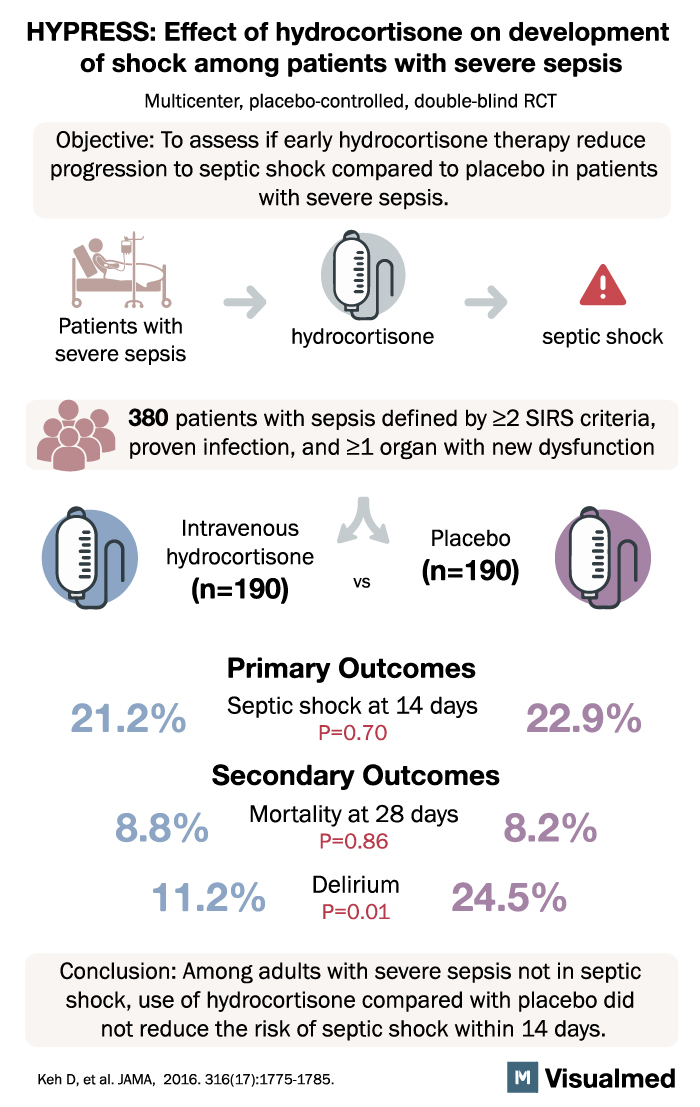
The HYPRESS trial was a randomized, double-blind, placebo-controlled trial designed to evaluate the effect of hydrocortisone in patients with sepsis but without septic shock. The primary outcome was the development of septic shock within 14 days.
Results from the HYPRESS trial showed that hydrocortisone did not prevent the development of septic shock in sepsis patients without shock at baseline. This study suggested that the routine use of steroids in sepsis without shock may not provide significant clinical benefits.
4. The ADRENAL Trial (2018)
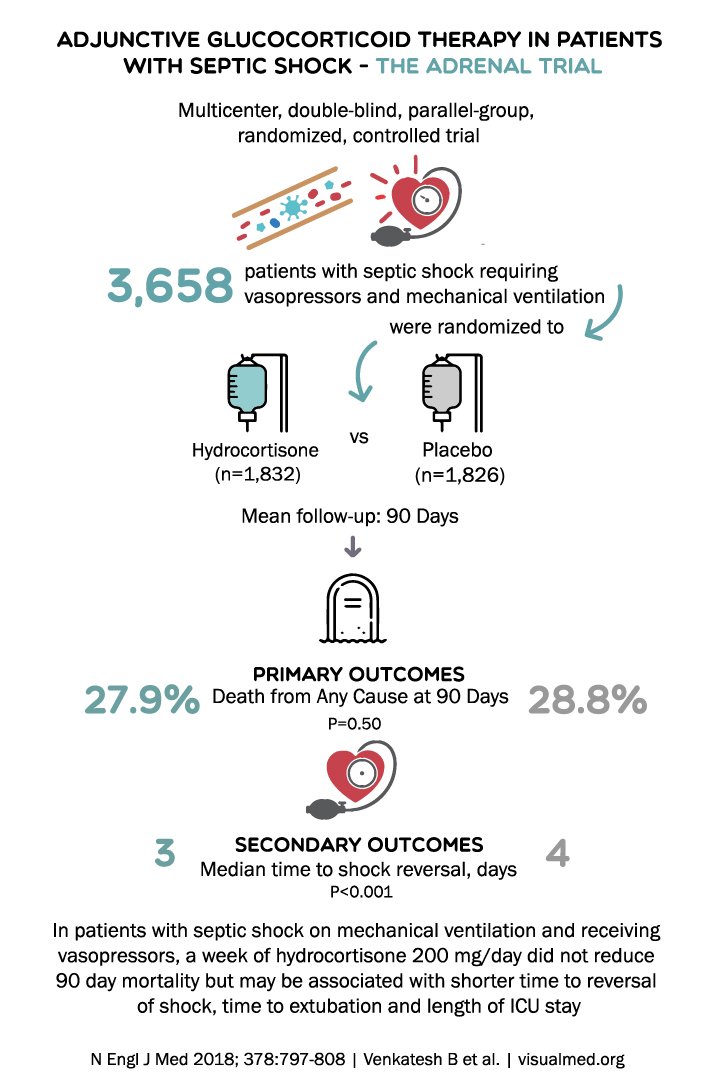
The ADRENAL trial was a multicenter, randomized, double-blind, placebo-controlled trial conducted to assess the efficacy of hydrocortisone in reducing 90-day all-cause mortality in critically ill patients with septic shock. The primary outcome was 90-day all-cause mortality.
The ADRENAL trial found no significant difference in 90-day mortality between the hydrocortisone group and the placebo group. However, the hydrocortisone group experienced a faster resolution of shock and a shorter duration of mechanical ventilation, suggesting that steroids may provide some clinical benefits in septic shock management.
5. The APROCCHSS Trial (2018)
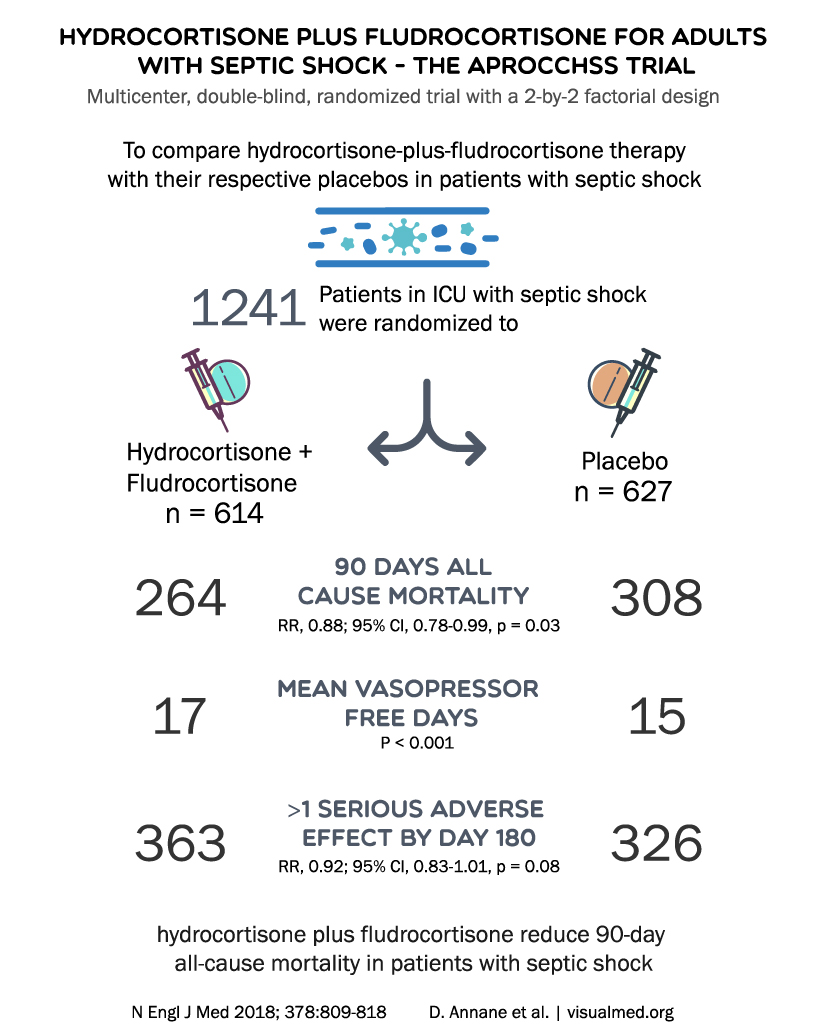
The APROCCHSS trial was a randomized, double-blind, placebo-controlled trial evaluating the effect of hydrocortisone plus fludrocortisone on 90-day all-cause mortality in septic shock patients. The primary outcome was 90-day all-cause mortality.
Results from the APROCCHSS trial demonstrated that the combination of hydrocortisone and fludrocortisone significantly reduced 90-day mortality in septic shock patients compared to placebo. This study provided evidence supporting the use of combined corticosteroid therapy in septic shock management, highlighting the potential benefits of a more comprehensive approach to steroid administration.
6. The VASST Trial (2009)
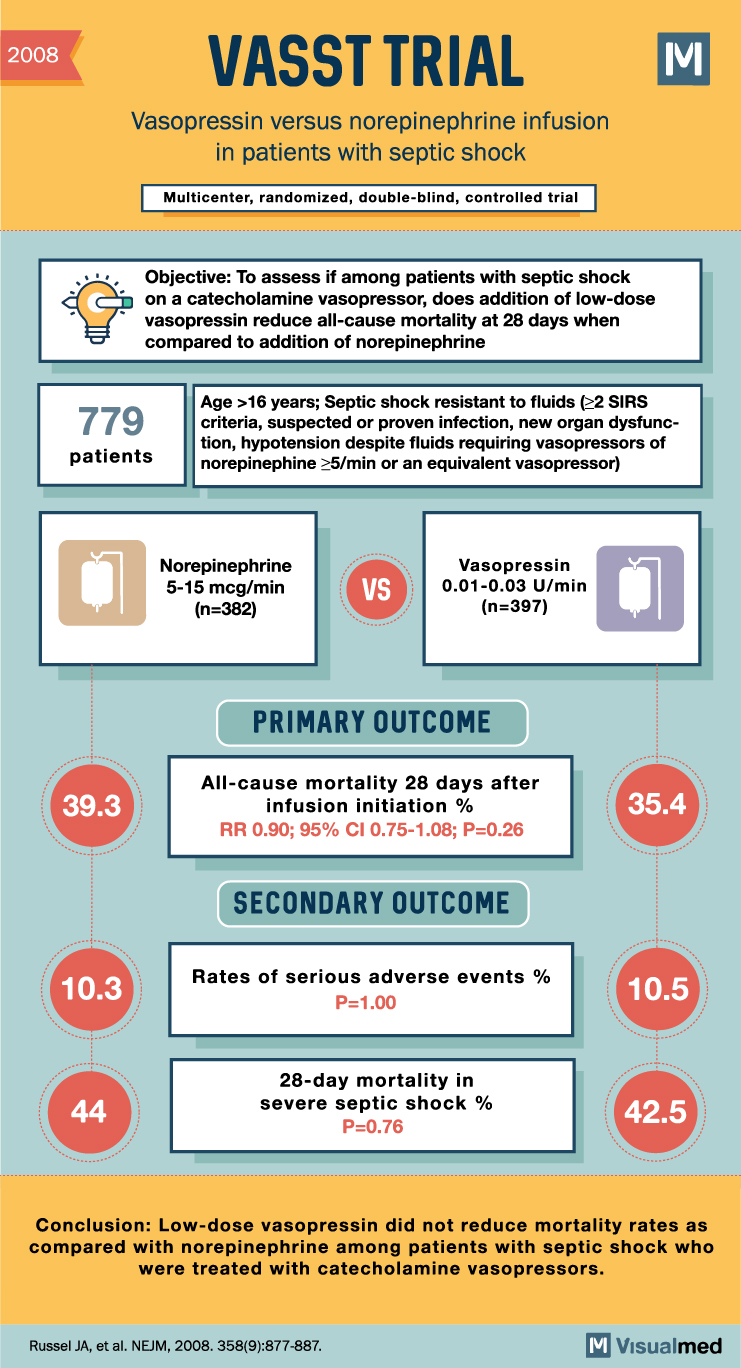
The Vasopressin and Septic Shock Trial (VASST) was a randomized, double-blind, placebo-controlled trial that investigated the use of low-dose vasopressin in addition to corticosteroids in septic shock patients. The primary outcome was 28-day all-cause mortality.
Although the VASST trial primarily focused on vasopressin, it provided valuable information on the impact of corticosteroids in septic shock management. The trial found no significant difference in mortality between patients receiving vasopressin plus corticosteroids and those receiving norepinephrine plus corticosteroids.
7. The SEPSISPAM Trial (2014)
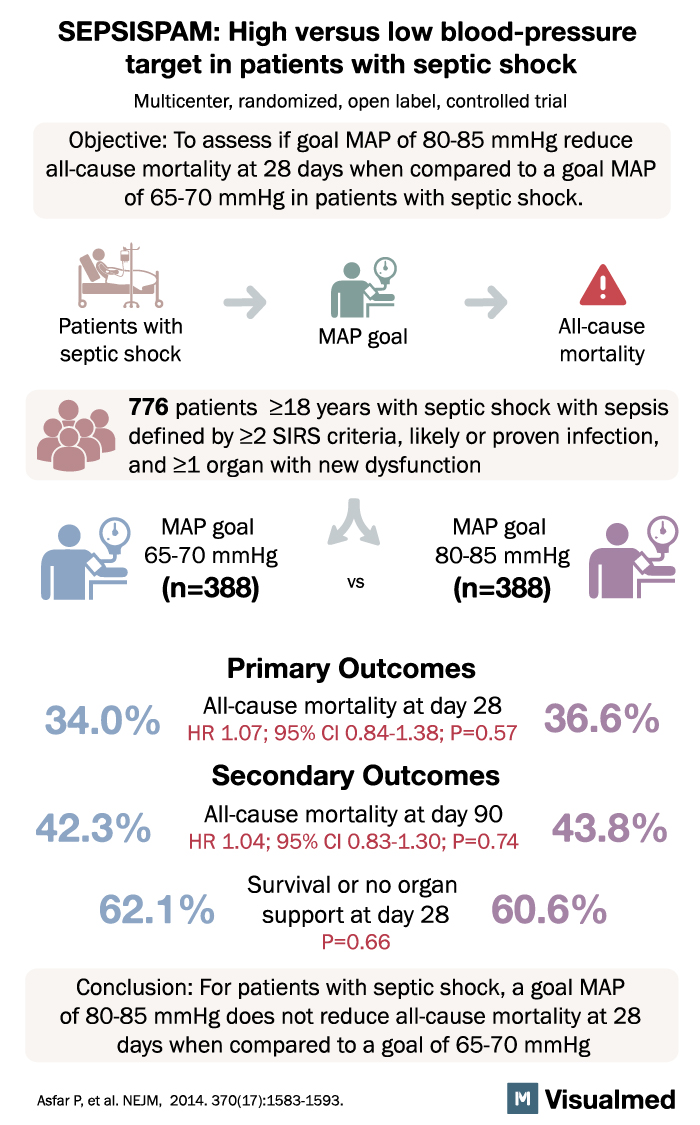
The SEPSISPAM trial was a multicenter, randomized, double-blind, placebo-controlled study that aimed to evaluate the effect of early administration of drotrecogin alfa (activated) and hydrocortisone in patients with septic shock. The primary outcome was 28-day all-cause mortality.
Results from the SEPSISPAM trial showed no significant difference in 28-day mortality between patients receiving early administration of drotrecogin alfa and hydrocortisone and those receiving standard care. This study added to the body of evidence suggesting that the benefits of corticosteroids in septic shock management may be limited in certain patient populations and clinical situations.
8. The COIITSS Trial (2012)
The COIITSS trial was a randomized, double-blind, placebo-controlled study designed to assess the efficacy of low-dose hydrocortisone in combination with intensive insulin therapy in patients with severe sepsis and septic shock. The primary outcome was the time to shock reversal.
Results from the COIITSS trial demonstrated that low-dose hydrocortisone combined with intensive insulin therapy significantly reduced the time to shock reversal compared to placebo. This study provided further support for the potential benefits of corticosteroids in septic shock management, particularly when used in combination with other therapeutic interventions.
Conclusion
The use of steroids in septic shock management remains a complex and controversial topic. Landmark clinical trials such as RALES, CORTICUS, HYPRESS, ADRENAL, and APROCCHSS have provided valuable insights into the potential benefits and limitations of steroid therapy in this critically ill patient population. While some trials have demonstrated significant mortality reduction and clinical benefits with steroid use, others have not found a clear advantage.
These discrepancies highlight the importance of tailoring treatment strategies to individual patients and specific clinical situations. Future research should focus on identifying patient subgroups that may derive the most significant benefits from steroid therapy and further refining treatment protocols to optimize patient outcomes. By building on the knowledge gained from these landmark trials, clinicians can better navigate the complexities of steroid use in septic shock management and make informed decisions to improve patient care.