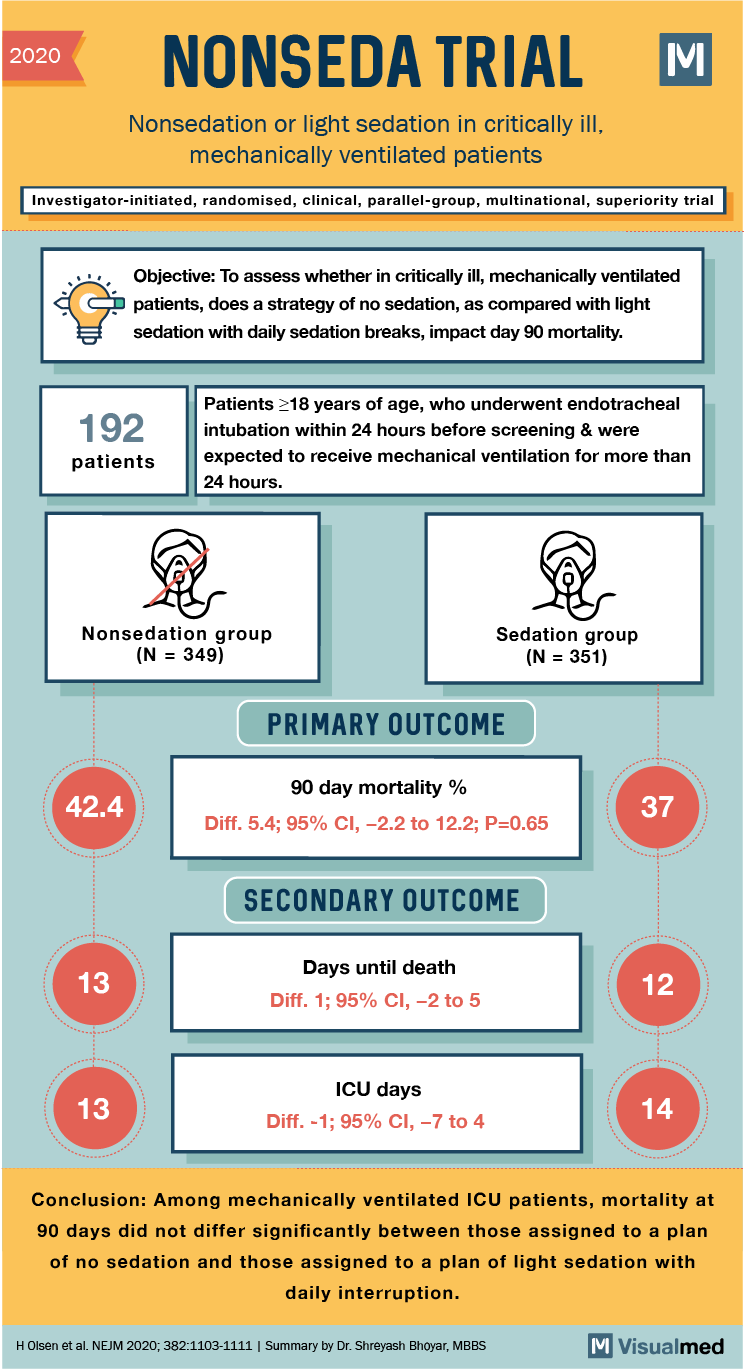
2020 NONSEDA TRIAL M Nonsedation or light sedation in critically ill, mechanically ventilated patients Investigator-initiated, randomised, clinical, parallel-group, multinational, superiority trial . a 5 Objective: To assess whether in critically ill, mechanically ventilated patients, does a strategy of no sedation, as compared with light sedation with daily sedation breaks, impact day 90 mortality. 192 patients Patients 18 years of age, who underwent endotracheal intubation within 24 hours before screening & were expected to receive mechanical ventilation for more than 24 hours. Nonsedation group (N = 349) Sedation group (N = 351) | PRIMARY OUTCOME 42.4 90 day mortality % Diff. 5.4; 95% CI, -2.2 to 12.2; P=0.65 SECONDARY OUTCOME Days until death Diff. 1; 95% CI, -2 to 5 12 13 ICU days Diff. – 1; 95% CI, -7 to 4 Conclusion: Among mechanically ventilated ICU patients, mortality at 90 days did not differ significantly between those assigned to a plan of no sedation and those assigned to a plan of light sedation with daily interruption. H Olsen et al. NEJM 2020; 382:1103-1111