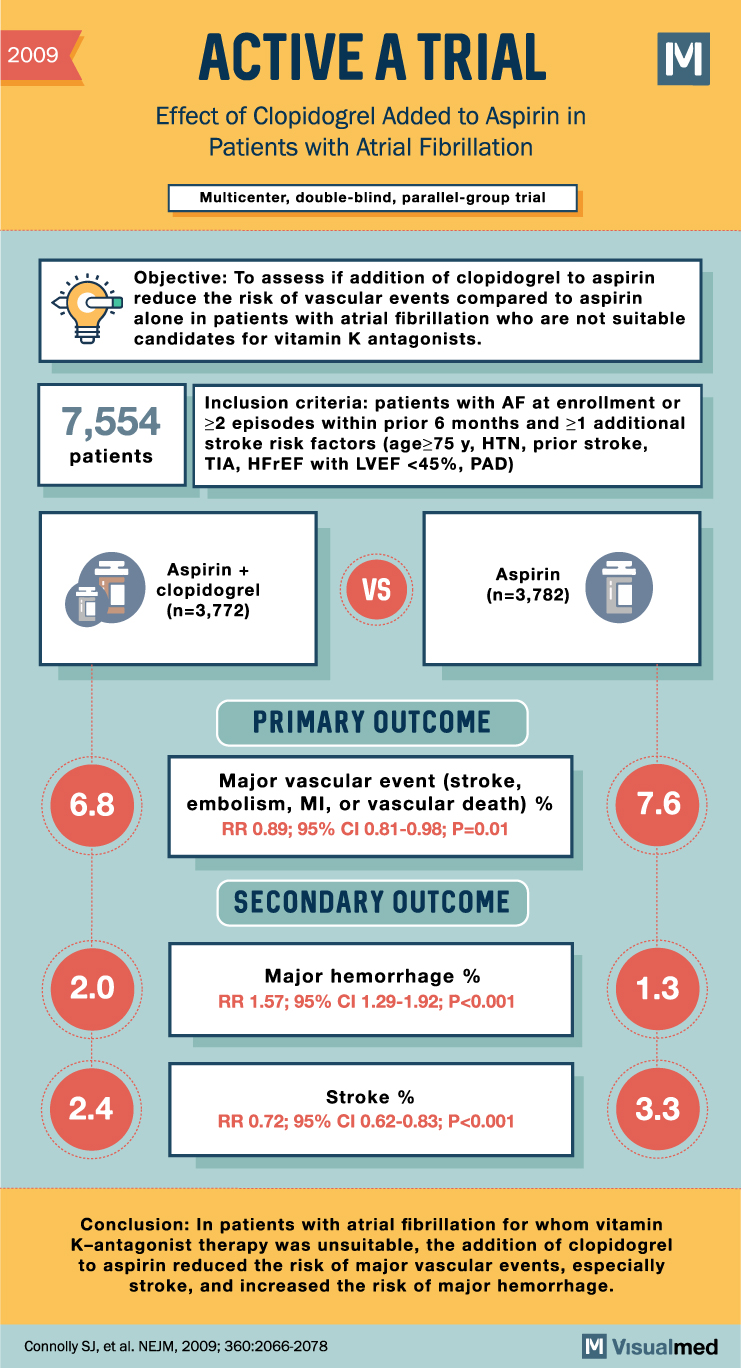
2009 ACTIVE A TRIAL Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation Multicenter, double-blind, parallel-group trial M Objective: To assess if the addition of clopidogrel to aspirin reduces the risk of vascular events compared to aspirin alone in patients with atrial fibrillation who are not suitable candidates for vitamin K antagonists. Inclusion criteria: patients with AF at enrollment or 7,554 22 episodes within prior 6 months and ≥1 additional stroke risk factors (age≥75 y, HTN, prior stroke, patients TIA, HFREF with LVEF <45%, PAD) A Aspirin + clopidogrel (n=3,772) VS Aspirin (n=3,782) PRIMARY OUTCOME 6.8 Major vascular event (stroke, embolism, MI, or vascular death) % RR 0.89; 95% CI 0.81-0.98; P=0.01 7.6 SECONDARY OUTCOME Major hemorrhage % 2.0 1.3 RR 1.57; 95% CI 1.29-1.92; P<0.001 2.4 Stroke % 3.3 RR 0.72; 95% CI 0.62-0.83; P<0.001 Conclusion: In patients with atrial fibrillation for whom vitamin K-antagonist therapy was unsuitable, the addition of clopidogrel to aspirin reduced the risk of major vascular events, especially stroke, and increased the risk of major hemorrhage. Connolly SJ, et al. NEJM, 2009; 360:2066-2078