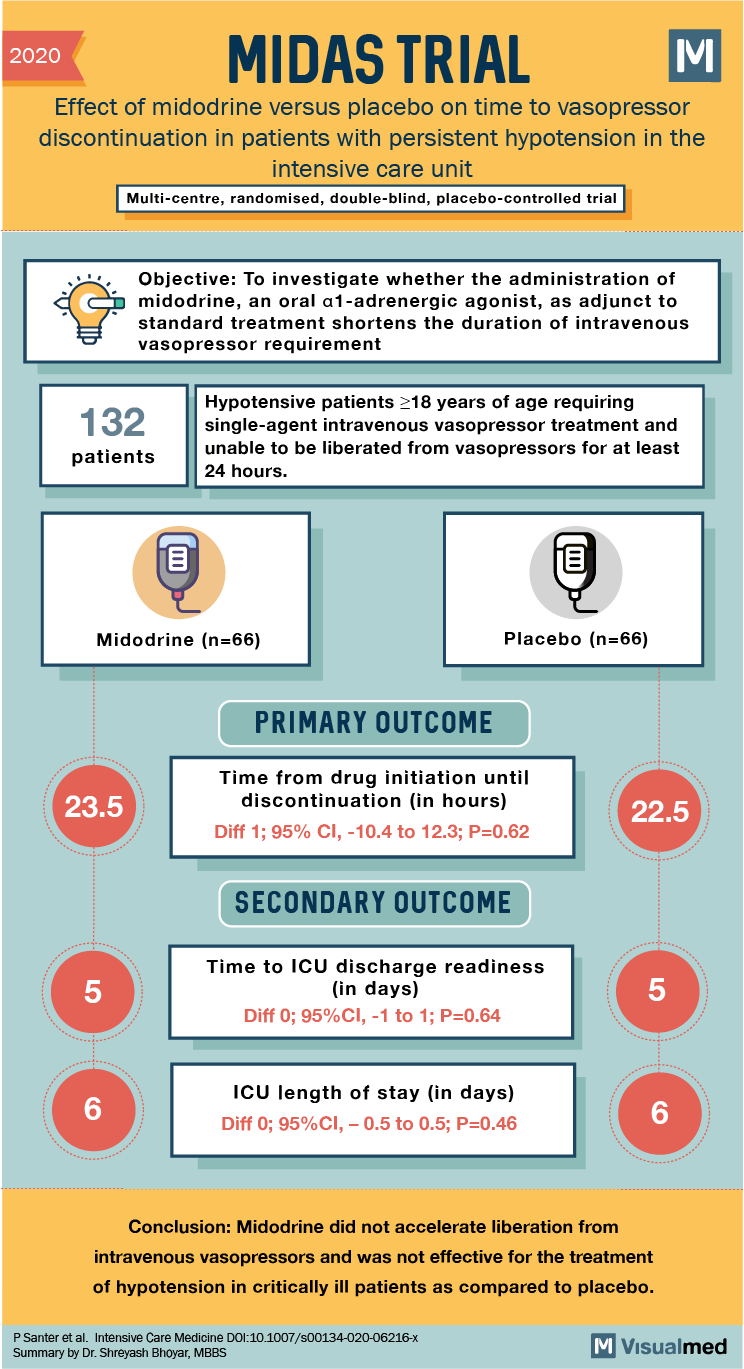
2020 MIDAS TRIAL: Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit Multi-centre, randomised, double-blind, placebo-controlled trial Objective: To investigate whether the administration of midodrine, an oral al-adrenergic agonist, as adjunct to standard treatment shortens the duration of intravenous vasopressor requirement 132 Hypotensive patients >18 years of age requiring single-agent intravenous vasopressor treatment and unable to be liberated from vasopressors for at least 24 hours. patients Midodrine (n=66) Placebo (n=66) PRIMARY OUTCOME 23.5 Time from drug initiation until discontinuation in hours) Diff 1; 95% CI, -10.4 to 12.3; P=0.62 22.5 SECONDARY OUTCOME Time to ICU discharge readiness (in days) Diff 0; 95%CI, -1 to 1; P=0.64 lind ICU length of stay (in days) Diff 0; 95%CI, -0.5 to 0.5; P=0.46 Conclusion: Midodrine did not accelerate liberation from intravenous vasopressors and was not effective for the treatment of hypotension in critically ill patients as compared to placebo. P Santer et al. Intensive Care Medicine DOI:10.1007/s00134-020-06216-X