Here’s the extracted information from the provided visual abstract: SOURCE Adjuvant Transarterial Chemoembolization With Sorafenib for Portal Vein Tumor Thrombus Study Design: Objective: Participants: Intervention Groups: Primary Outcome: Secondary Outcomes: Conclusion: Citation: This summary captures the key points from the … Read More
Oncology

CheckMate 77T Trial: Nivolumab in Lung Cancer
CheckMate 77TPerioperative Nivolumab in Resectable Lung Cancer Design: Objective: Patients: Inclusion criteria: Exclusion criteria: Comparison: Primary Outcome: Secondary Outcomes: Conclusion: Reference: This data highlights the key points of the CheckMate 77T trial, comparing the outcomes of perioperative nivolumab combined with … Read More
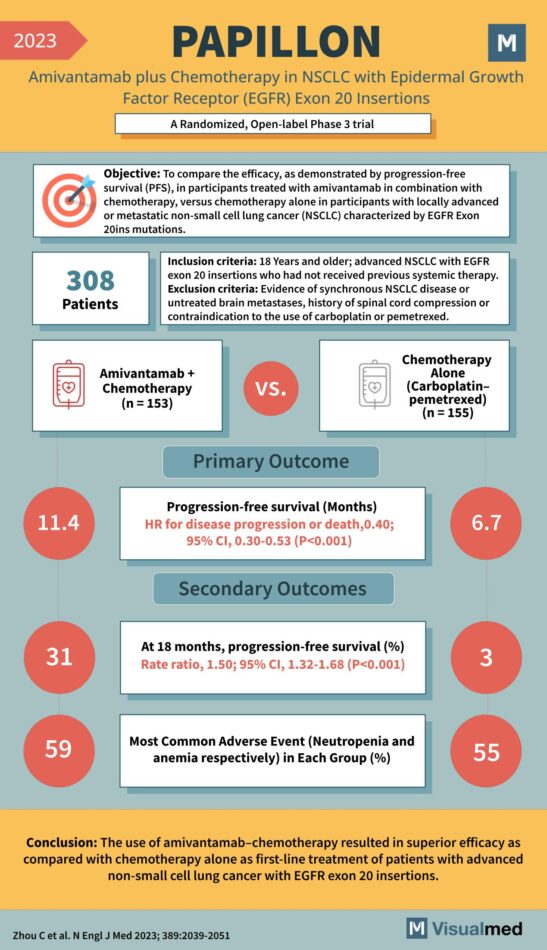
PAPILLON Trial: Amivantamab in Lung Cancer
Year: 2023 Title: PAPILLON Subtitle: Amivantamab plus Chemotherapy in NSCLC with Epidermal Growth Factor Receptor (EGFR) Exon 20 Insertions Type of Trial: A Randomized, Open-label Phase 3 trial Objective: To compare the efficacy, as demonstrated by progression-free survival (PFS), in … Read More
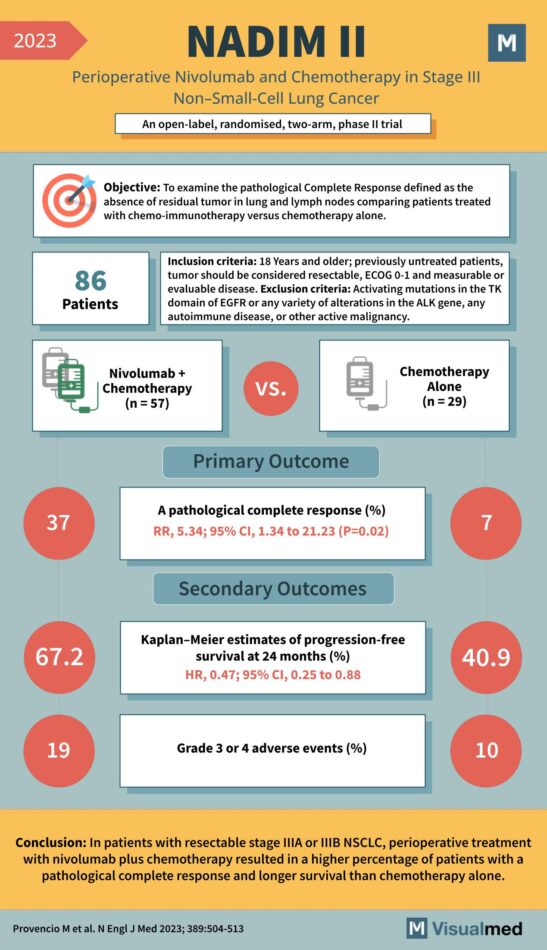
NADIM II Trial: Nivolumab in Lung Cancer
Objective: To examine the pathological Complete Response defined as the absence of residual tumor in lung and lymph nodes comparing patients treated with chemo-immunotherapy versus chemotherapy alone. Inclusion Criteria: Exclusion Criteria: Participants: 86 Patients Treatment Groups: Primary Outcome: Secondary Outcomes: … Read More
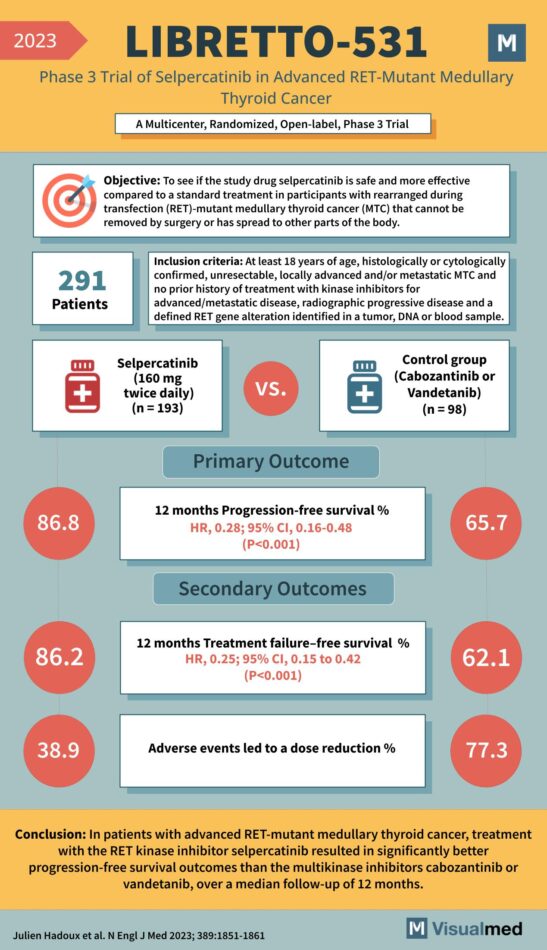
LIBRETTO-531 Trial: Selpercatinib in Medullay Thyroid Cancer
Objective: To determine if the study drug selpercatinib is safer and more effective compared to standard treatment in participants with RET-mutant medullary thyroid cancer (MTC) that cannot be removed by surgery or has spread to other parts of the body. … Read More
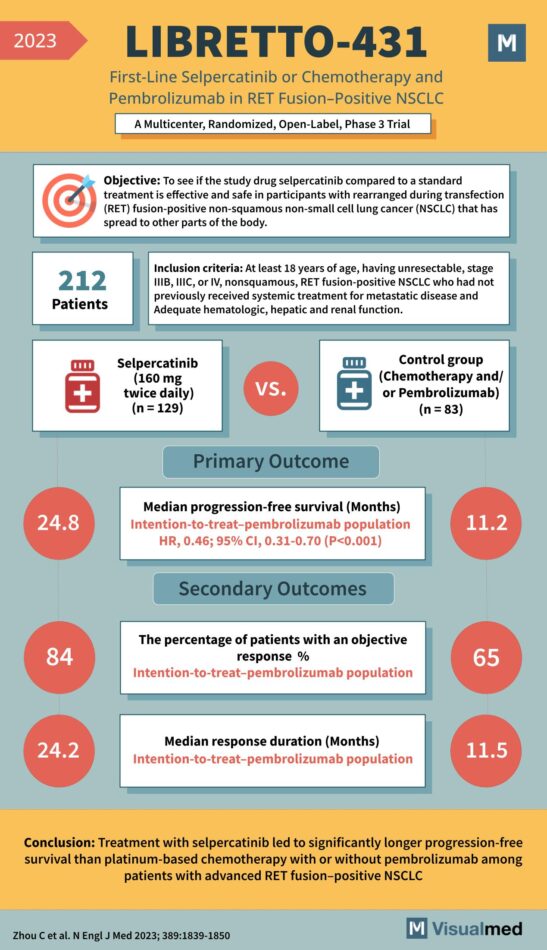
LIBRETTO-431 Trial: Selpercatinib in NSCLC
Objective: To determine if the study drug selpercatinib, compared to a standard treatment, is effective and safe in participants with RET fusion-positive non-squamous non-small cell lung cancer (NSCLC) that has spread to other parts of the body. Inclusion Criteria: Participants: … Read More
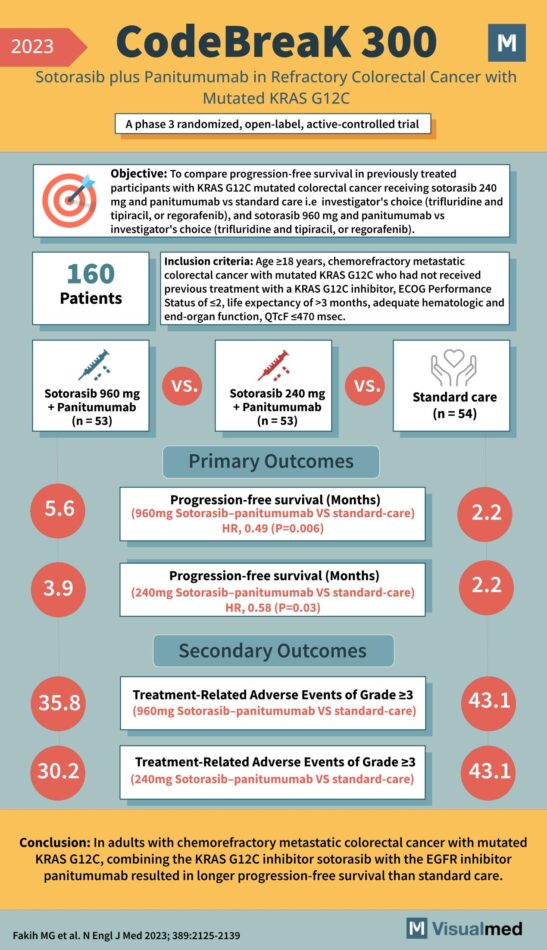
CodeBreak 300 Trial: Sotorasib + Panitumumab in CRC
Year: 2023 Title: CodeBreaK 300 Subtitle: Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C Type of Trial: A phase 3 randomized, open-label, active-controlled trial Objective: To compare progression-free survival in previously treated participants with KRAS G12C mutated … Read More
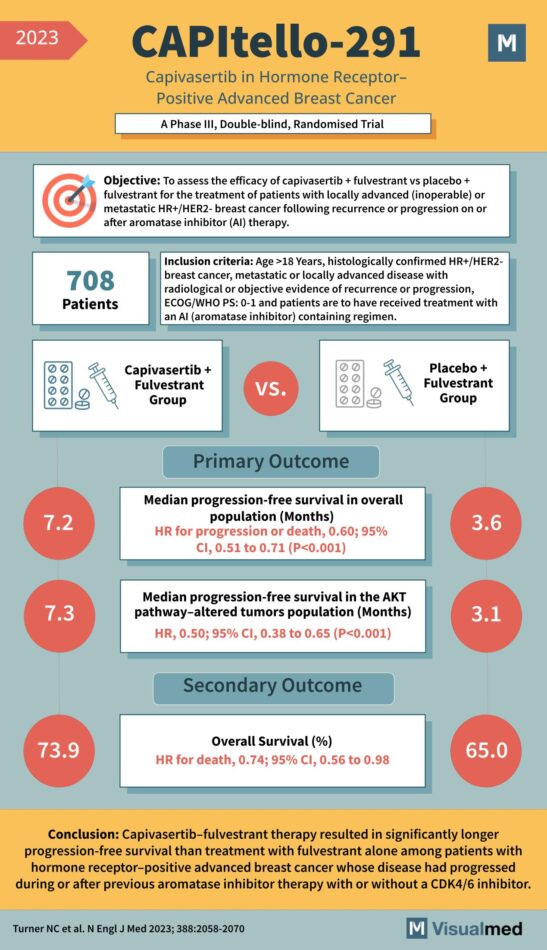
CAPItello-291: Capivasertib in Breast Cancer
The CAPItello-291 Trial: A New Horizon in Breast Cancer Treatment The CAPItello-291 Trial, a Phase III double-blind randomized trial conducted in 2023, has significantly contributed to the evolving landscape of breast cancer treatment, particularly for hormone receptor-positive advanced cases. Objective … Read More
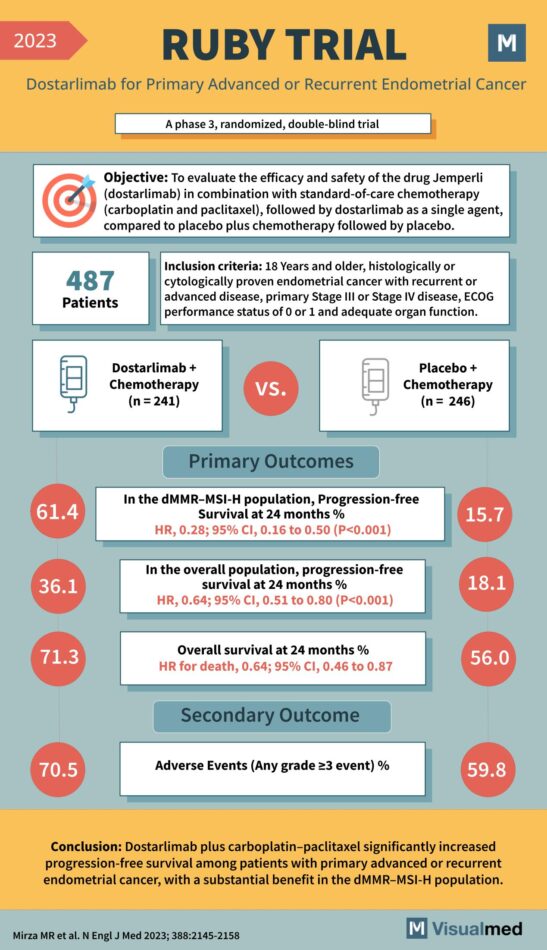
RUBY Trial Summary: Dostarlimab for Endometrial Cancer
The RUBY Trial: A New Chapter in Endometrial Cancer Treatment Recent results from the RUBY Trial have introduced a significant advancement in the treatment of primary advanced or recurrent endometrial cancer. The phase 3, randomized, double-blind trial meticulously evaluated the … Read More
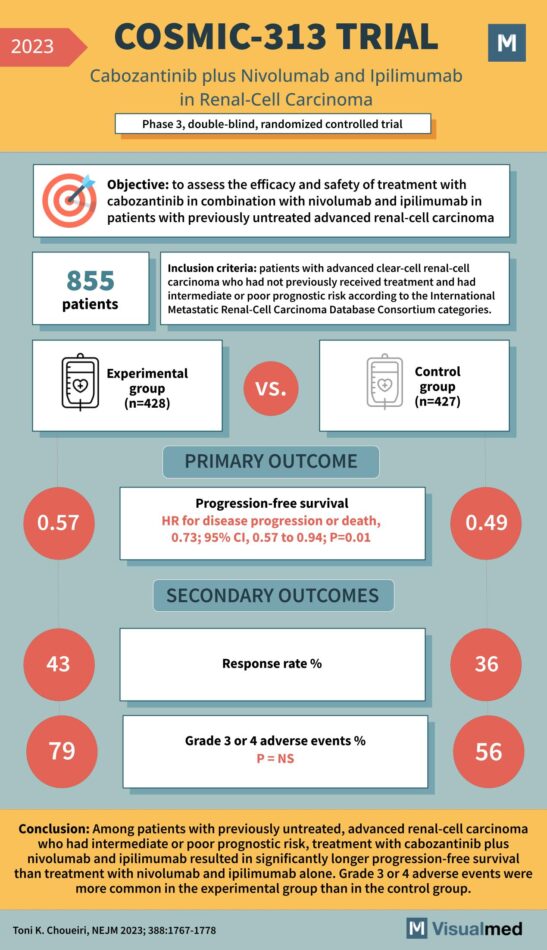
COSMIC-313 Trial: Cabozantinib in Renal Cell Cancer
The COSMIC-313 Trial: A New Frontier in Renal-Cell Carcinoma Treatment In the landscape of oncology, renal-cell carcinoma stands as a formidable challenge, often presenting at advanced stages and with a dire need for effective treatments. Enter the COSMIC-313 Trial, a … Read More
