Here’s the extracted information from the provided visual abstract: SABATO Efficacy and Safety of an Early Oral Switch in Low-risk Staphylococcus aureus Bloodstream Infection Study Design: Objective: Participants: Intervention Groups: Primary Outcome: Secondary Outcome: Conclusion: Citation: This summary captures the … Read More
Infectious Diseases

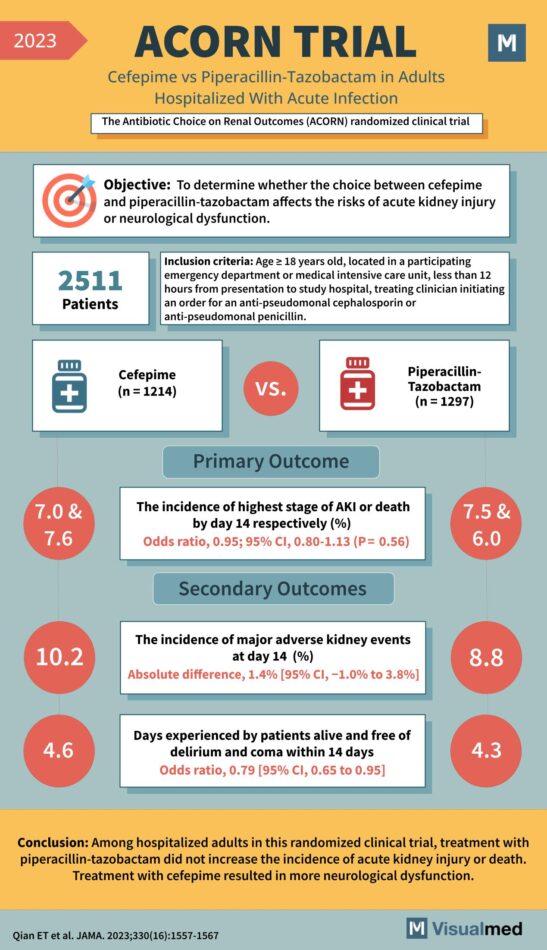
ACORN Trial: Cefepime vs. Piperacillin-Tazobactam
The ACORN Trial: Cefepime vs. Piperacillin-Tazobactam’s Impact on Renal Outcomes In 2023, the ACORN Trial set out to investigate the renal and neurological outcomes of hospitalized adults with acute infections treated with two common antibiotics, providing valuable insights for healthcare … Read More
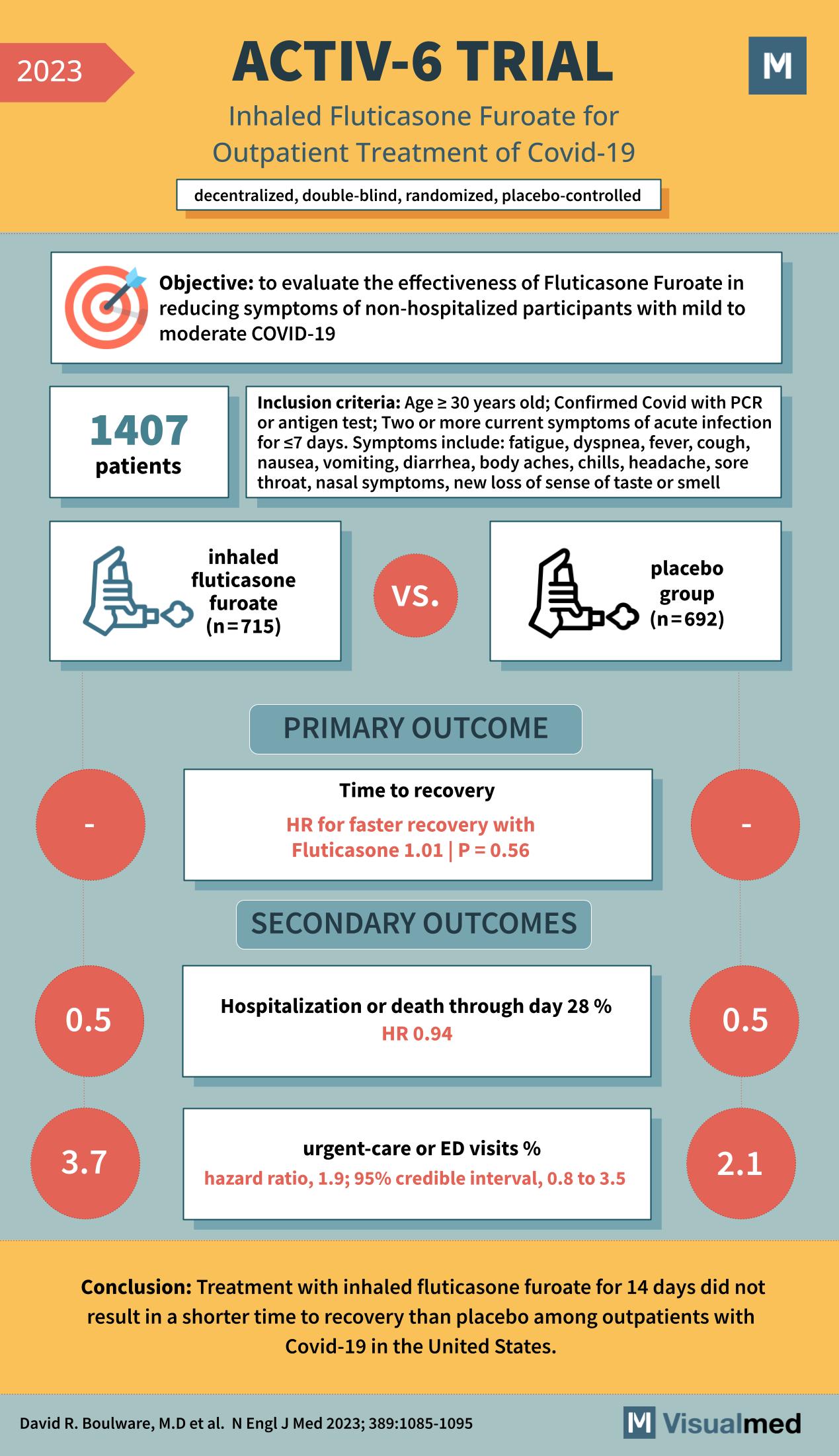
ACTIV-6 Trial: Inhaled Fluticasone in Covid-19
The ACTIV-6 trial, published in the New England Journal of Medicine in 2023, examines the effectiveness of inhaled fluticasone furoate in the treatment of non-hospitalized patients with mild to moderate COVID-19. The trial was a decentralized, double-blind, randomized, placebo-controlled study … Read More
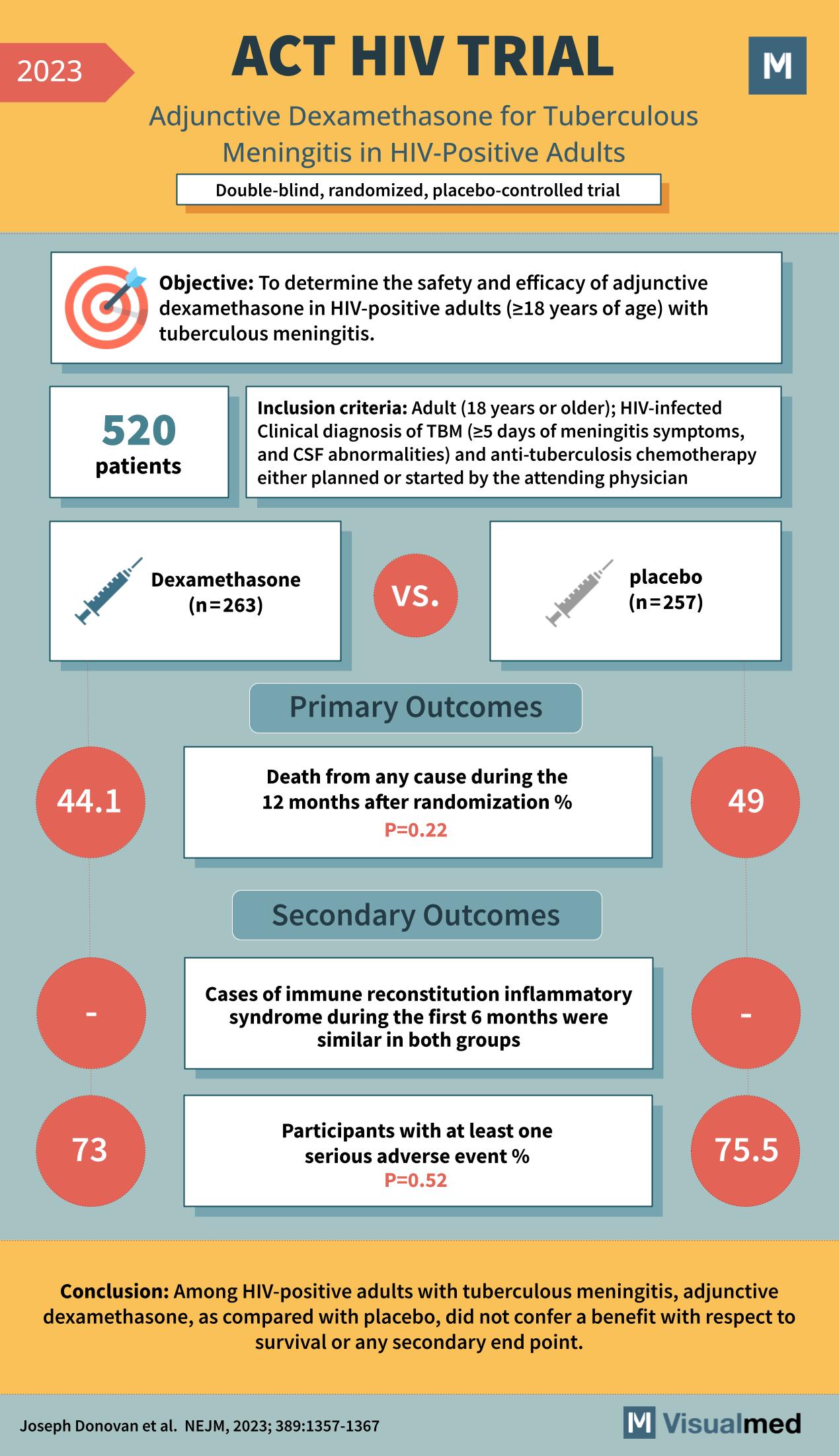
ACT HIV Trial: Dexamethasone in TB Meningitis
ACT HIV TRIAL (2023) Summary and Key Points In 2023, a significant study titled the “ACT HIV TRIAL” was published, which delved into the potential benefits of using adjunctive dexamethasone for the treatment of tuberculous meningitis in HIV-positive adults. This … Read More
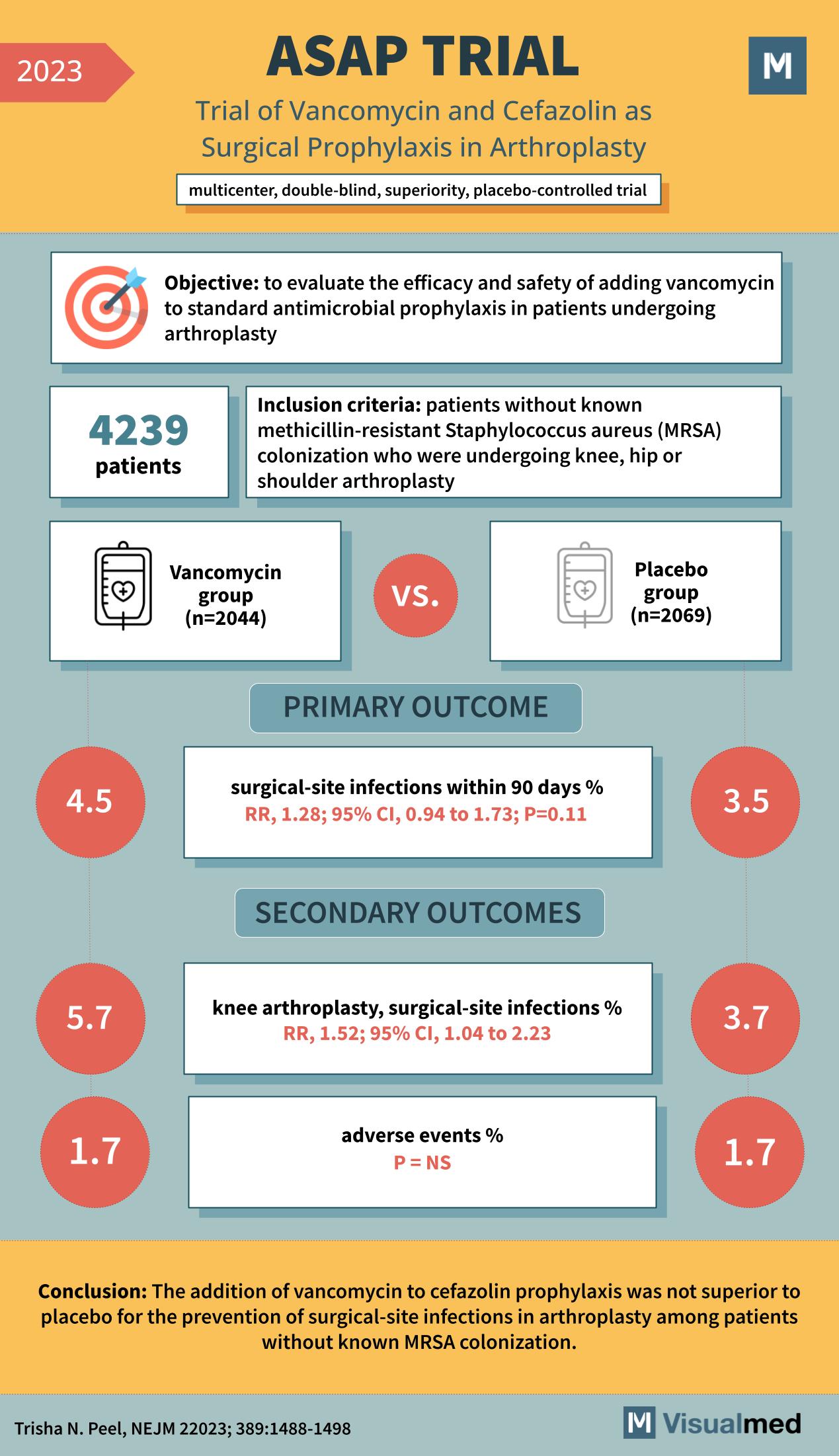
ASAP Trial: Vancomycin and Cefazolin in Arthroplasty Prophylaxis
The ASAP Trial: Vancomycin and Cefazolin in Arthroplasty Prophylaxis – An Analysis The realm of surgical prophylaxis has always been a topic of deep medical interest, aiming to prevent complications and ensure the best outcomes for patients. One such endeavor … Read More
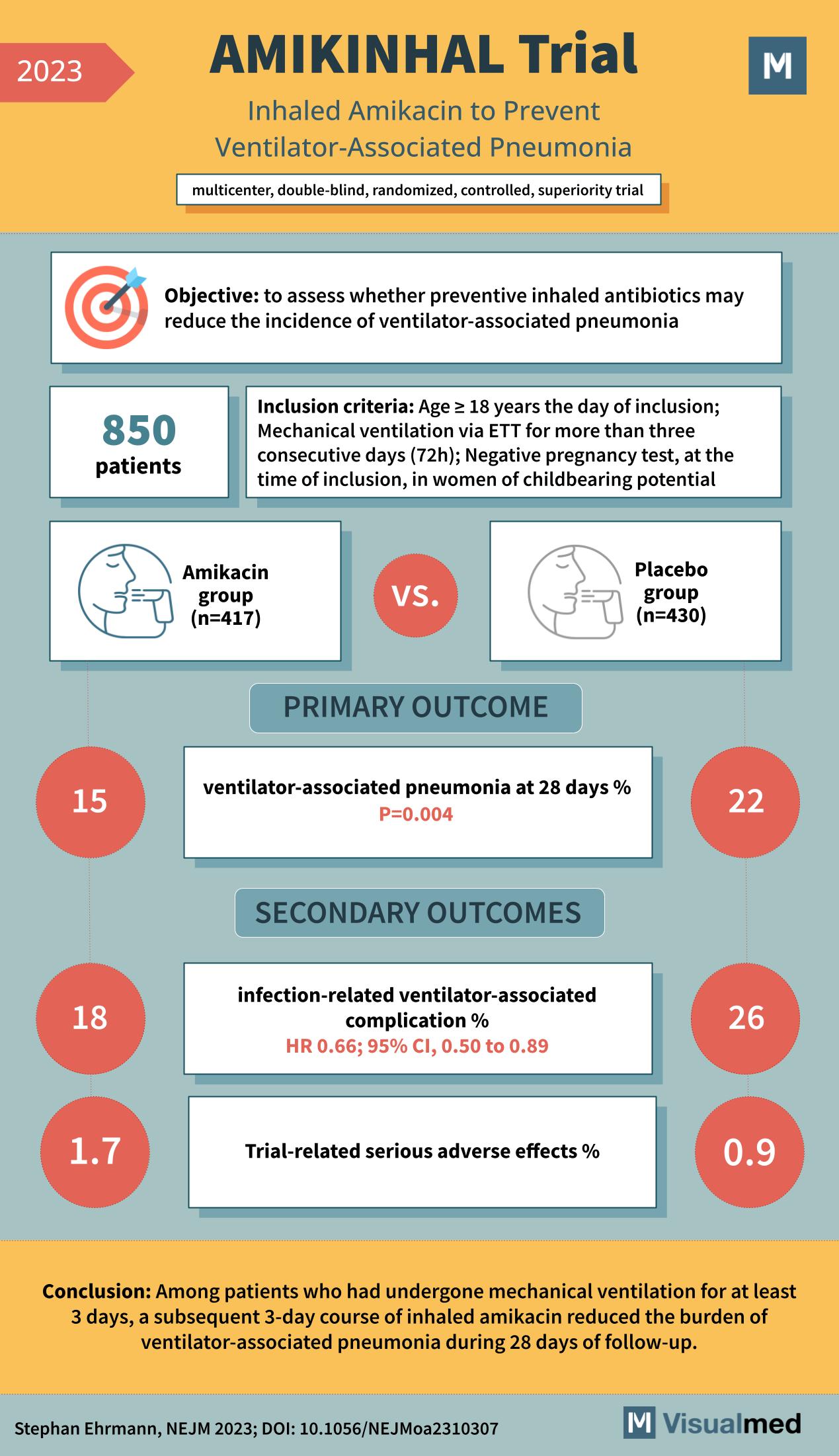
AMIKINHAL Trial: Inhaled Amikacin in Preventing VAP
The medical world is continually seeking innovative methods to combat and prevent life-threatening conditions. One such condition is ventilator-associated pneumonia, a severe complication that affects patients undergoing mechanical ventilation. The recent AMIKINHAL trial, a multicenter, double-blind, randomized, controlled, superiority study, … Read More
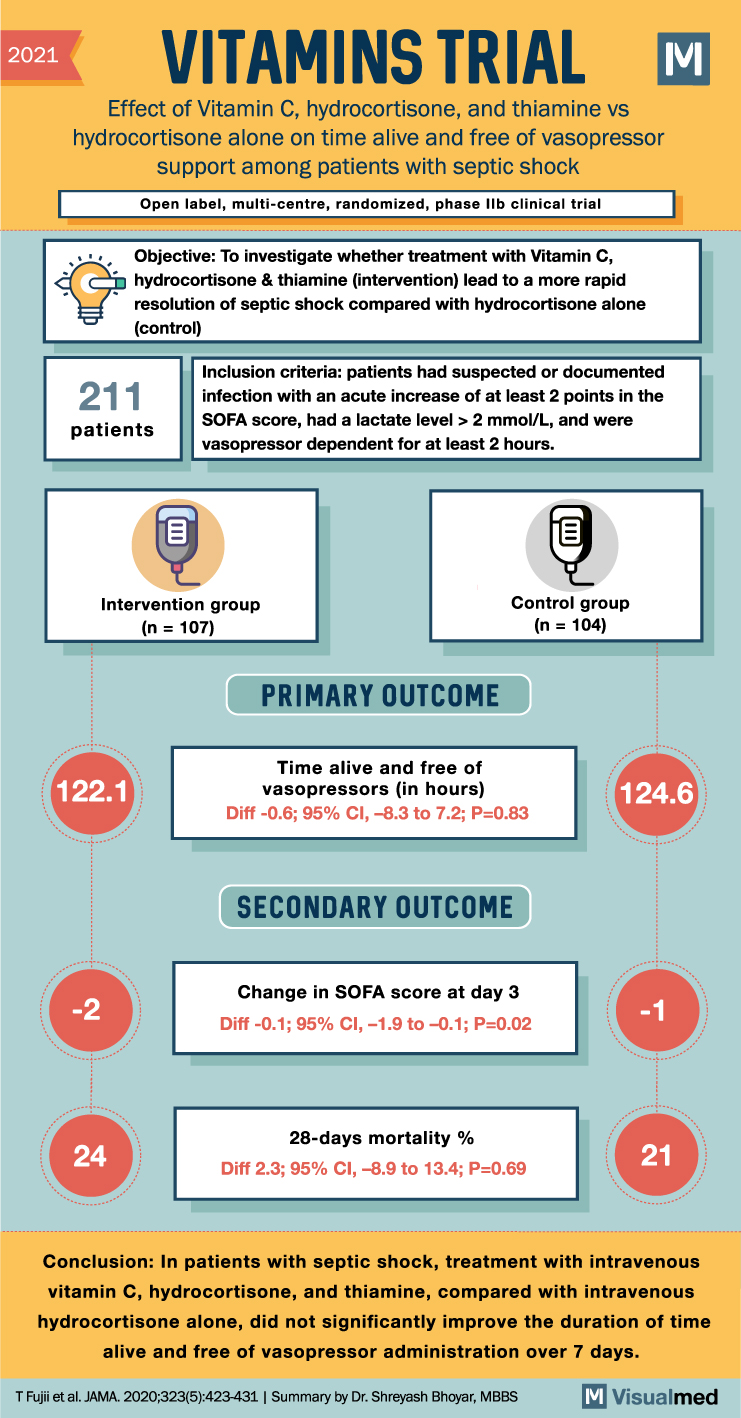
VITAMINS Trial Summary: Vit. C in Septic Shock
VITAMINS Trial Summary The VITAMINS trial aimed to determine whether the combination of vitamin C, hydrocortisone, and thiamine is more effective than hydrocortisone alone in expediting the resolution of septic shock. The trial included 216 patients with septic shock and … Read More
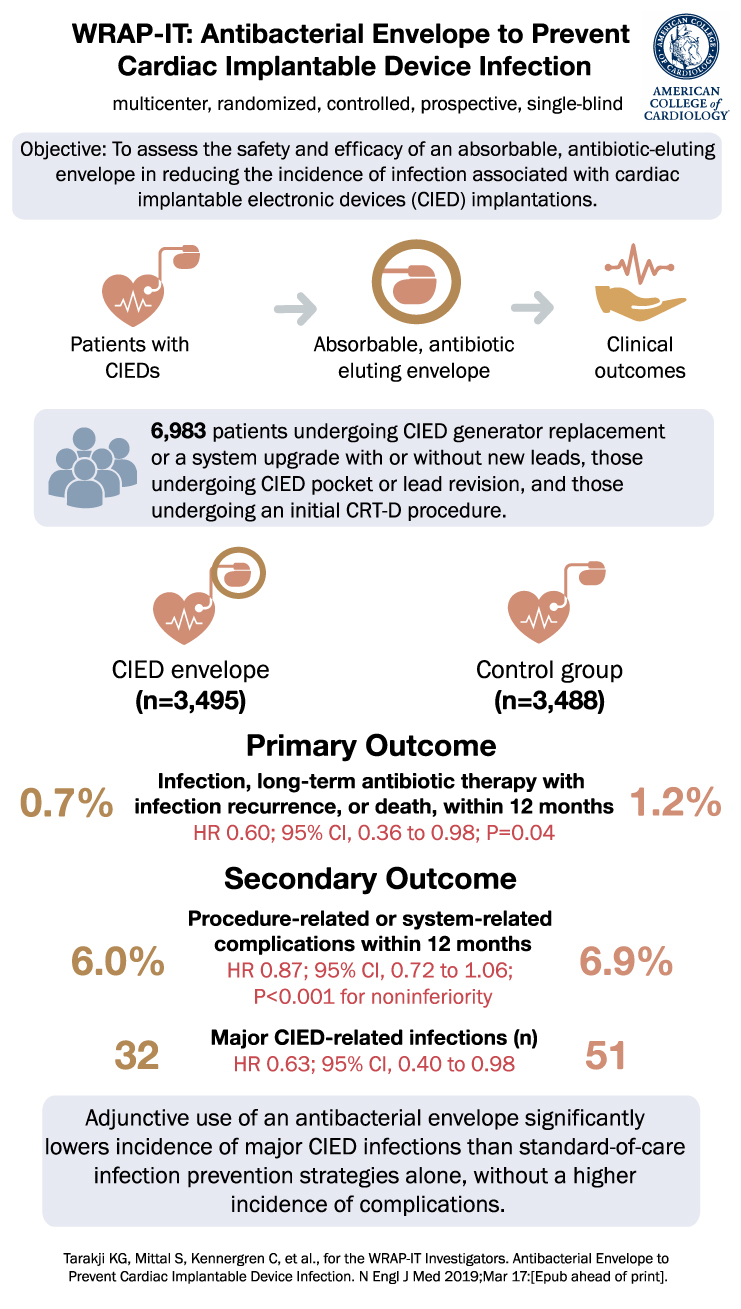
WRAP-IT Trial: Utilizing Antibiotic-Eluting Envelopes to Reduce Infections After Cardiac Device Implantation
WRAP-IT Trial Summary Infections following the placement of cardiac implantable electronic devices (CIEDs) can lead to significant morbidity and mortality. Although preoperative antibiotics are a standard prophylactic strategy, evidence for other preventive approaches is limited. The WRAP-IT trial sought to … Read More
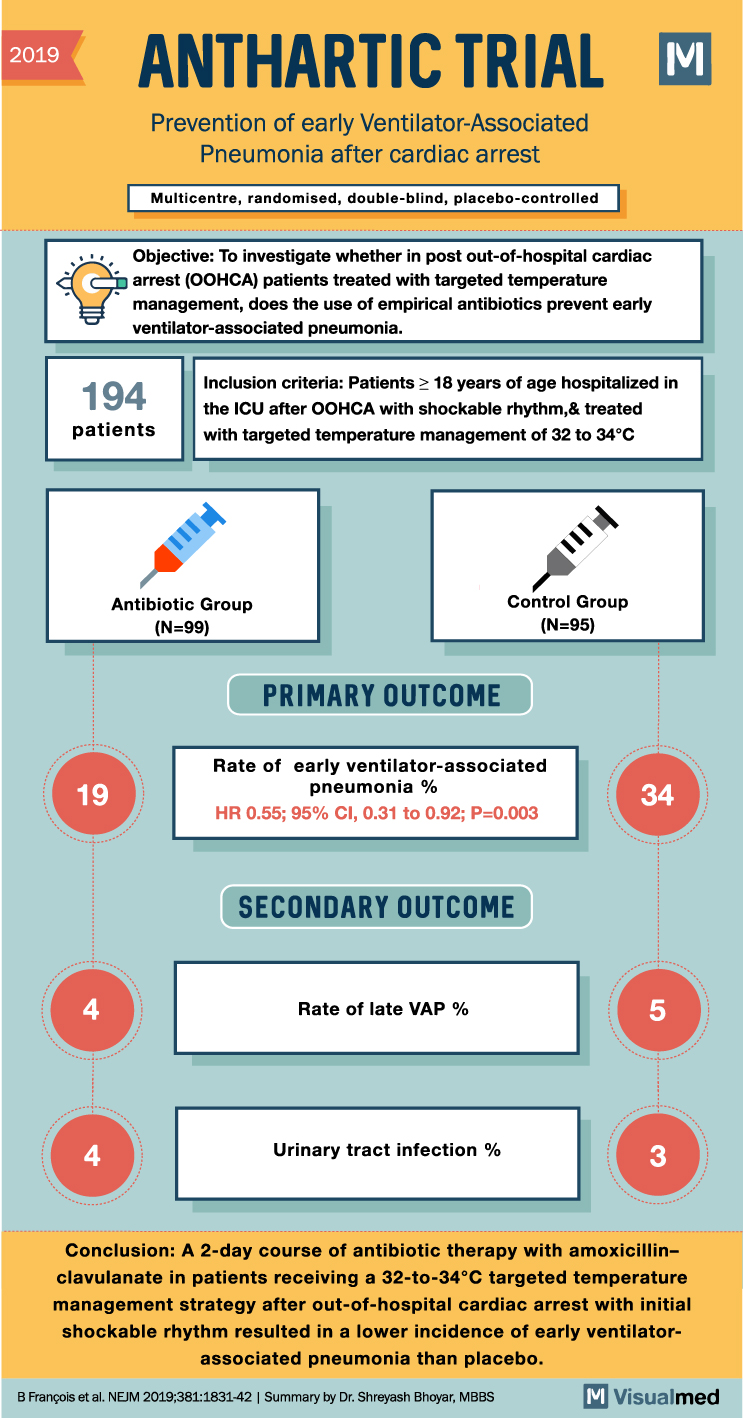
ANTHARTIC Trial: Preventing VAP after CA
2019 ANTHARTIC TRIAL M Prevention of early Ventilator-Associated Pneumonia after cardiac arrest Multicentre, randomised, double-blind, placebo-controlled Objective: To investigate whether in post out-of-hospital cardiac arrest (OOHCA) patients treated with targeted temperature management, does the use of empirical antibiotics prevent early … Read More
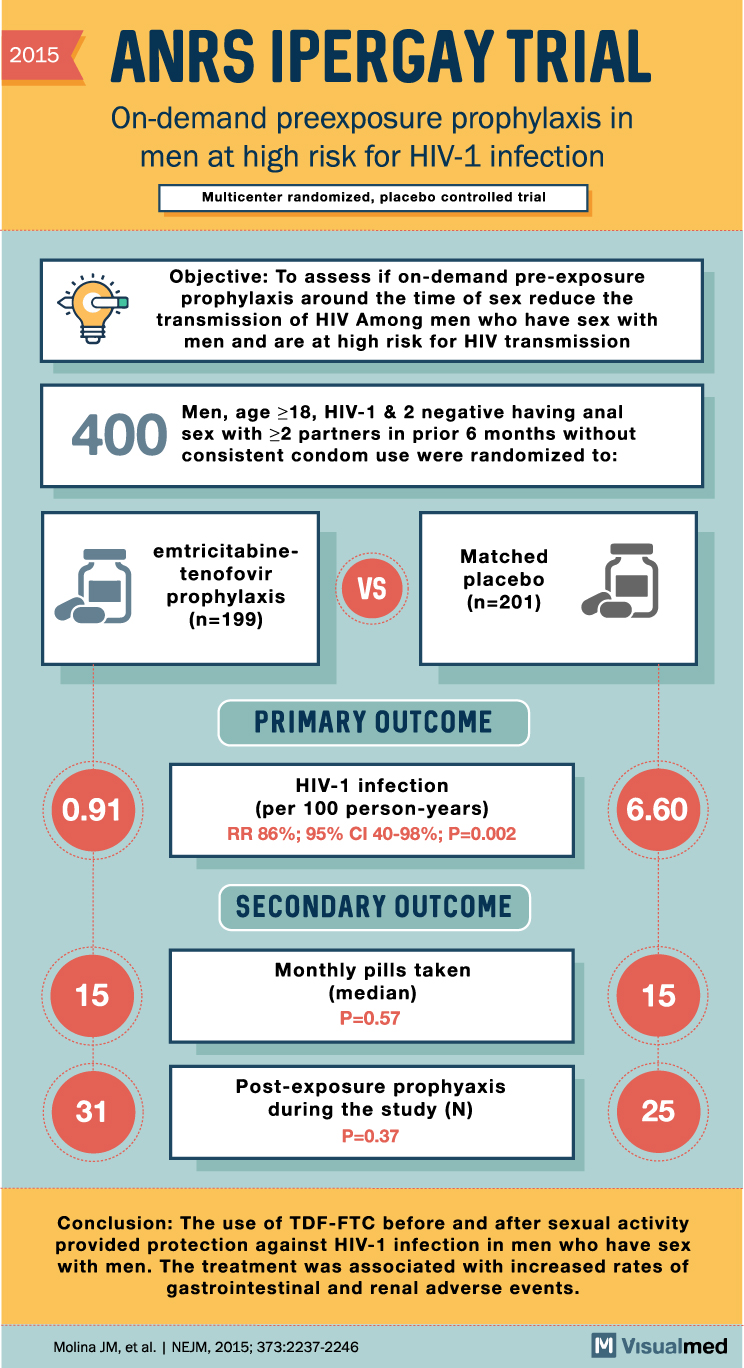
ANRS IPERGAY Trial: Prophylaxis in men at risk of HIV
2015 ANRS IPERGAY TRIAL On-demand preexposure prophylaxis in men at high risk for HIV-1 infection Multicenter randomized, placebo controlled trial Objective: To assess if on-demand pre-exposure prophylaxis around the time of sex reduce the transmission of HIV Among men who … Read More