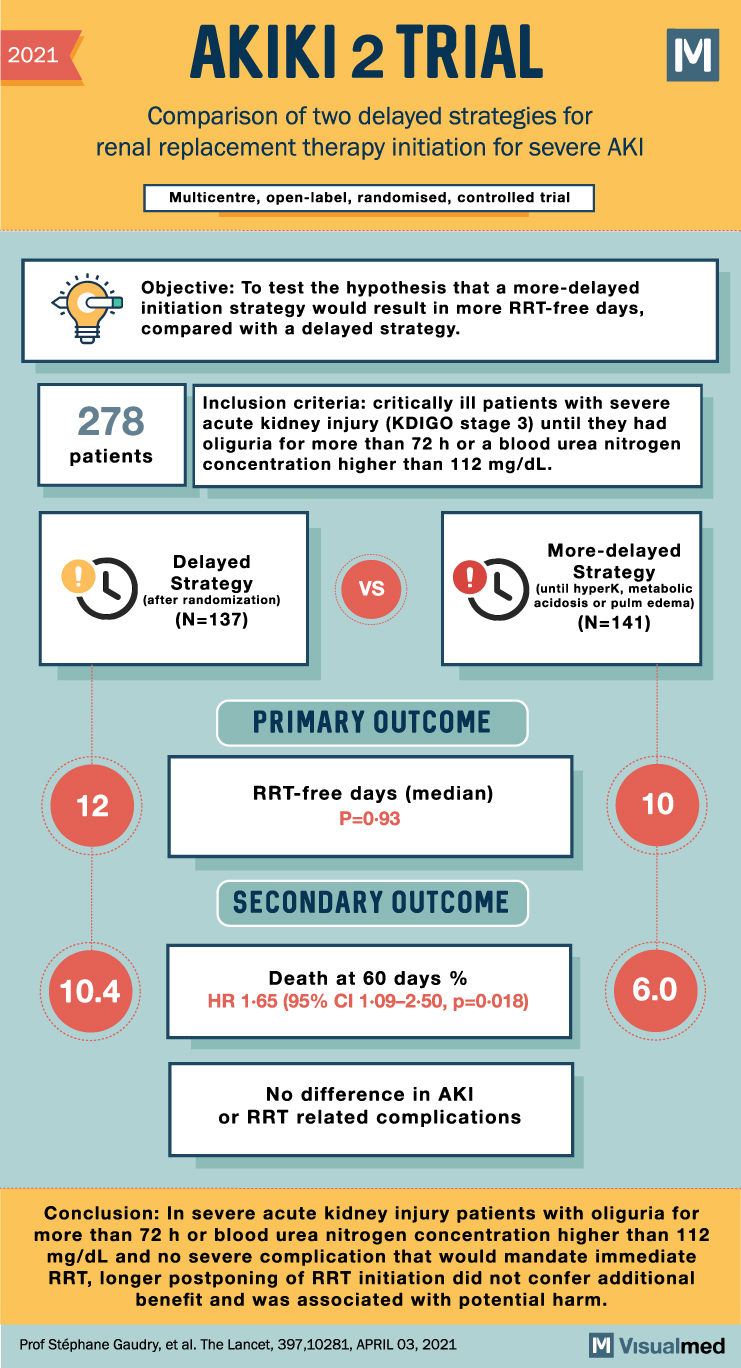
2021 AKIKI 2 TRIAL Comparison of two delayed strategies for renal replacement therapy initiation for severe AKI Multicentre, open-label, randomised, controlled trial Objective: To test the hypothesis that a more-delayed initiation strategy would result in more RRT-free days, compared with … Read More
Blog
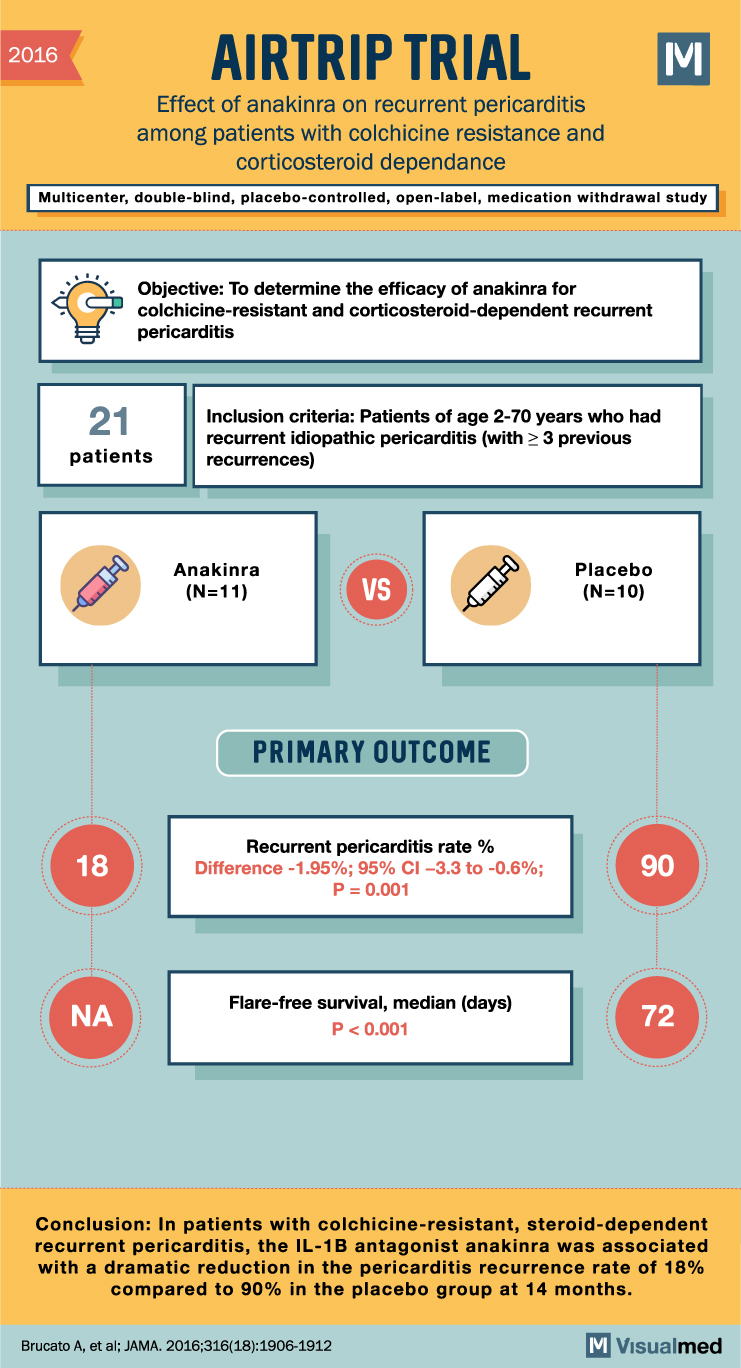
AIRTRIP Trial Summary: Anakinra in Recurrent Pericarditis
2016 AIRTRIP TRIAL Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and dependance M Multicenter, double-blind, placebo-controlled, open-label, medication withdrawal study Objective: To determine the efficacy of anakinra for colchicine-resistant and corticosteroid-dependent recurrent pericarditis 21 Inclusion criteria: … Read More
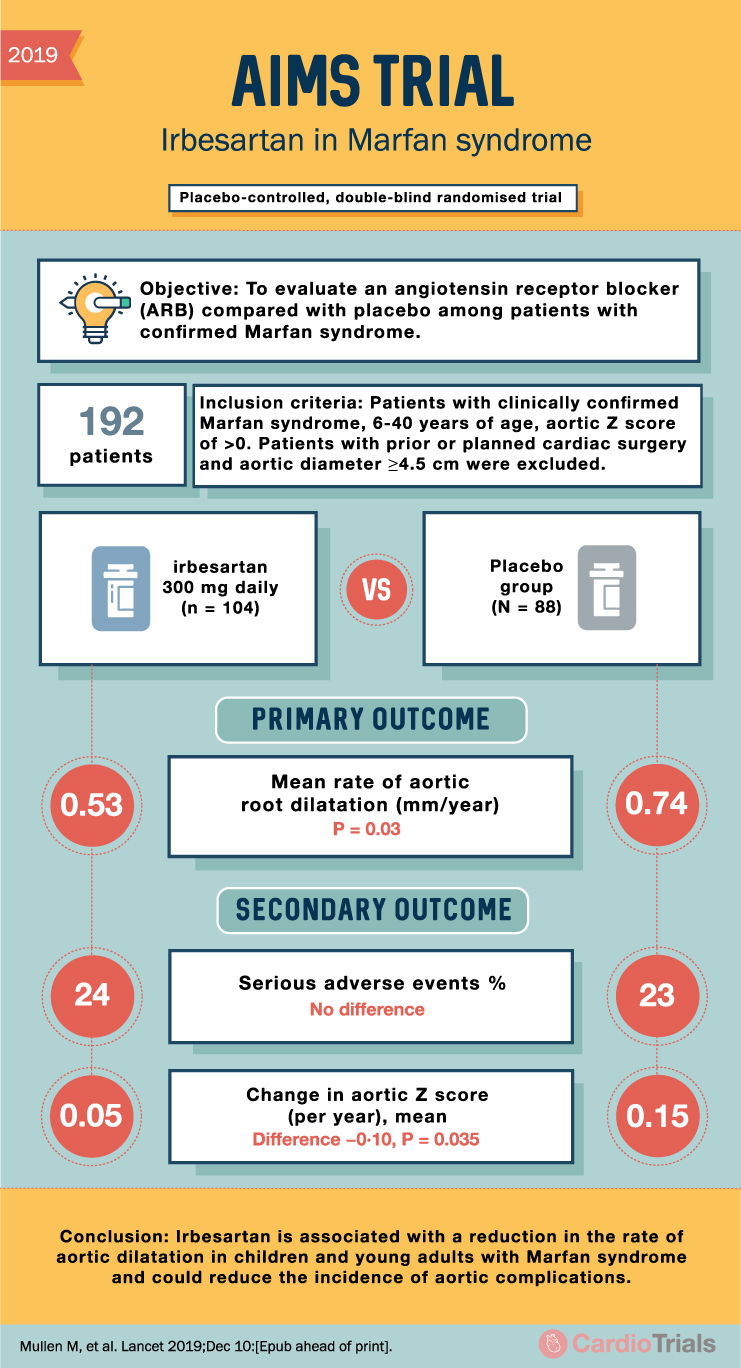
AIMS Trial Summary: Irbesartan in Marfan Syndrome
2019 AIMS TRIAL Irbesartan in Marfan syndrome Placebo-controlled, double-blind randomised trial Objective: To evaluate an angiotensin receptor blocker (ARB) compared with placebo among patients with confirmed Marfan syndrome. 192 patients Inclusion criteria: Patients with clinically confirmed Marfan syndrome, 6-40 years … Read More
AIM-HIGH Trial: Niacin + Statins in Hyperlipidemia
2011 AIM-HIGH TRIAL Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy Placebo controlled, randomized clinical trial Σ Objective: To assess if extended-release niacin added to simvastatin to raise low levels of high-density lipoprotein (HDL) cholesterol is … Read More
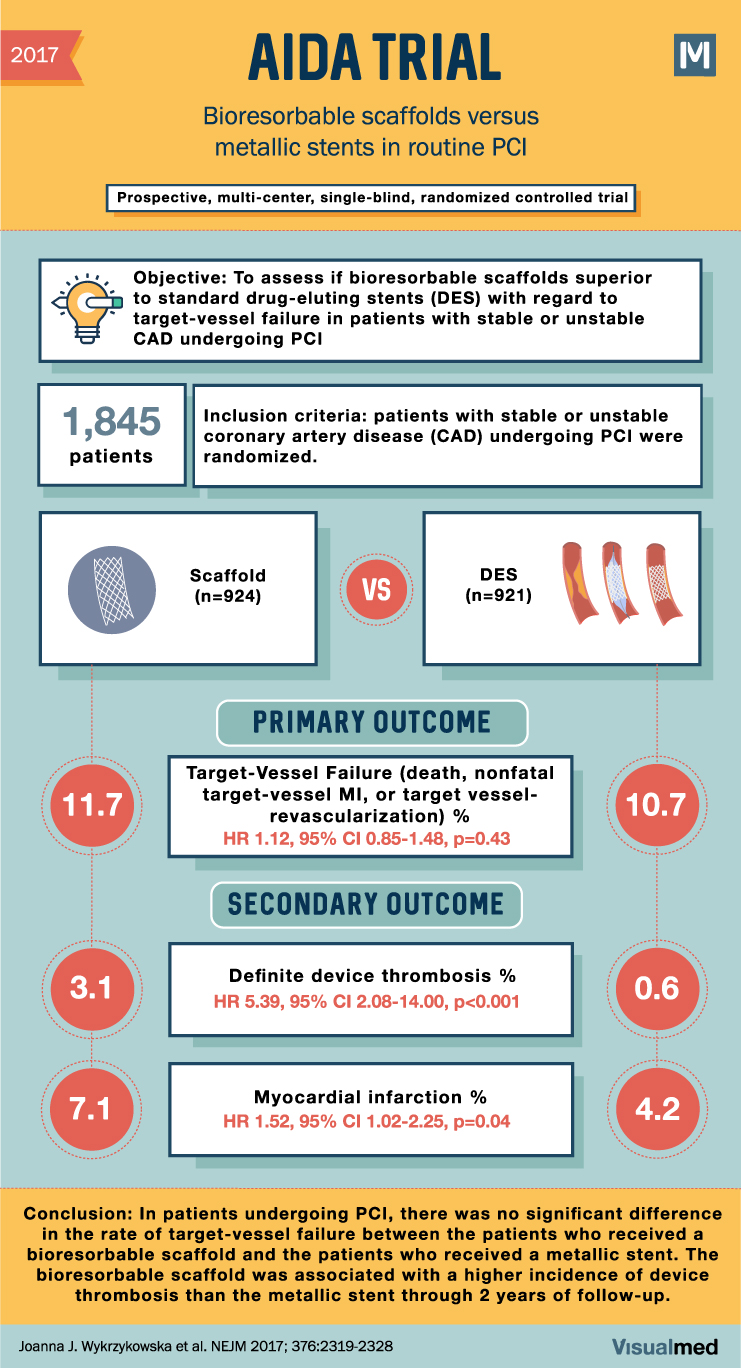
AIDA Trial Summary: Bioabsorbable Scaffolds for Routine PCI
2017 AIDA TRIAL Bioresorbable scaffolds versus metallic stents in routine PCI Prospective, multi-center, single-blind, randomized controlled trial M Objective: To assess if bioresorbable scaffolds superior to standard drug-eluting stents (DES) with regard to target-vessel failure in patients with stable or … Read More
AIDA-WP2 Trial Summary: Fosfomycin or Nitrofurantoin in UTI
2018 AIDA-WP2 Effect of 5-Day nitrofurantoin vs single-dose fosfomycin on M clinical resolution of uncomplicated lower urinary tract infection in women Multinational, open-label, analyst-blinded, randomized clinical trial Objective: To compare the clinical and microbiologic efficacy of nitrofurantoin and fosfomycin in … Read More
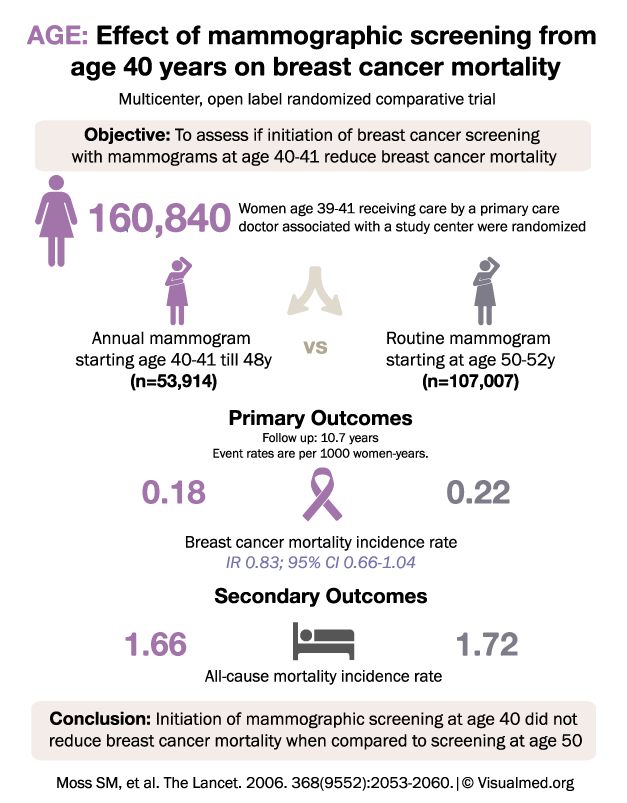
AGE Trial Summary: Mammogram Screening for Breast Cancer
AGE: Effect of mammographic screening from age 40 years on breast cancer mortality Multicenter, open label randomized comparative trial Objective: To assess if initiation of breast cancer screening with mammograms at age 40-41 reduces breast cancer mortality Women age 39-41 … Read More
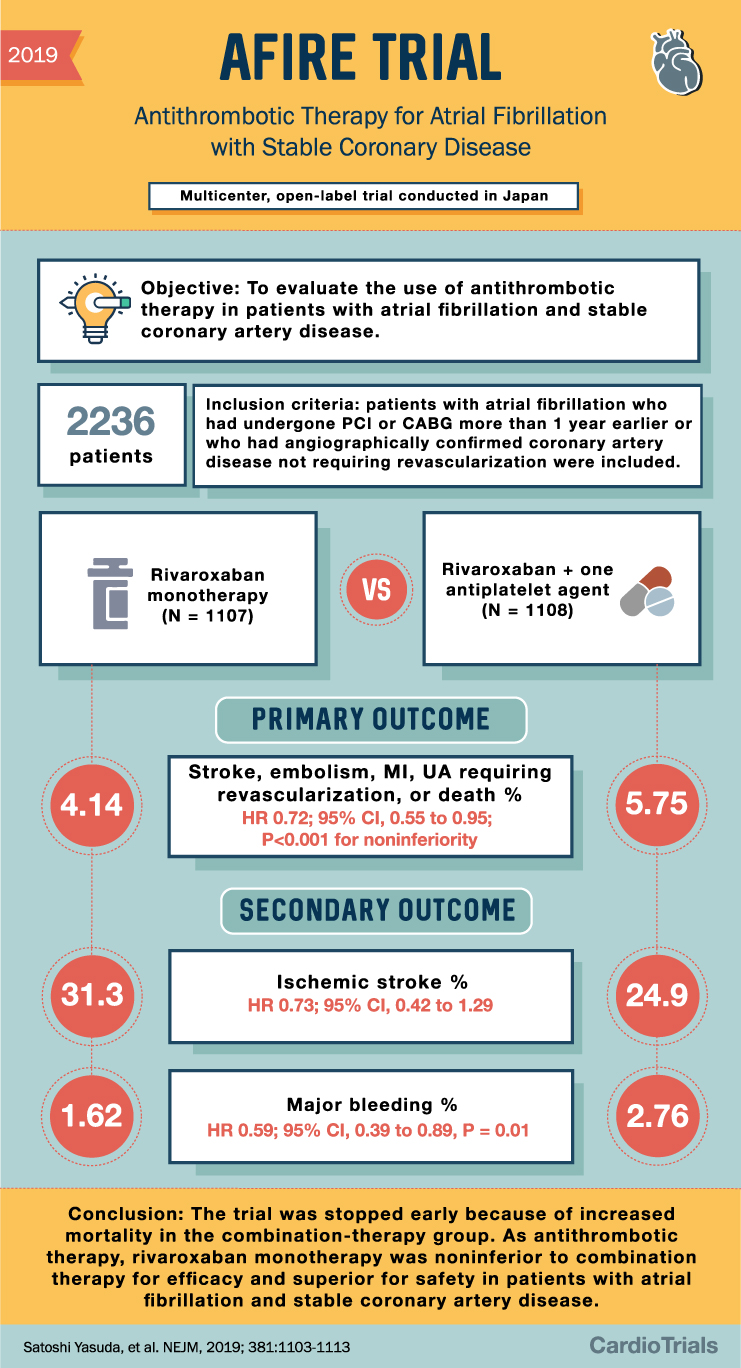
AFIRE Trial Summary: Antithrombotics in AFib and CAD
2019 AFIRE TRIAL Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease Multicenter, open-label trial conducted in Japan Objective: To evaluate the use of antithrombotic therapy in patients with atrial fibrillation and stable coronary artery disease. 2236 patients 4.14 Inclusion … Read More
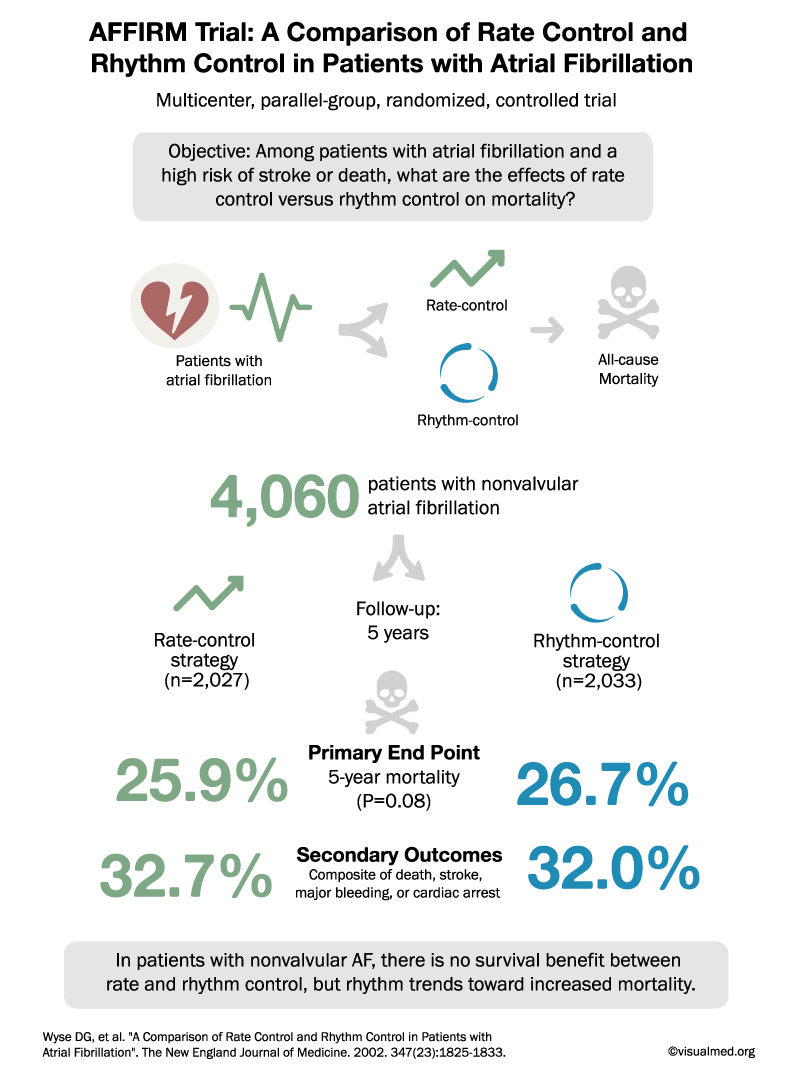
AFFIRM Trial Summary: Rate vs Rhythm Control for AFib
AFFIRM Trial: A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation Multicenter, parallel-group, randomized, controlled trial Objective: Among patients with atrial fibrillation and a high risk of stroke or death, what are the effects of rate … Read More
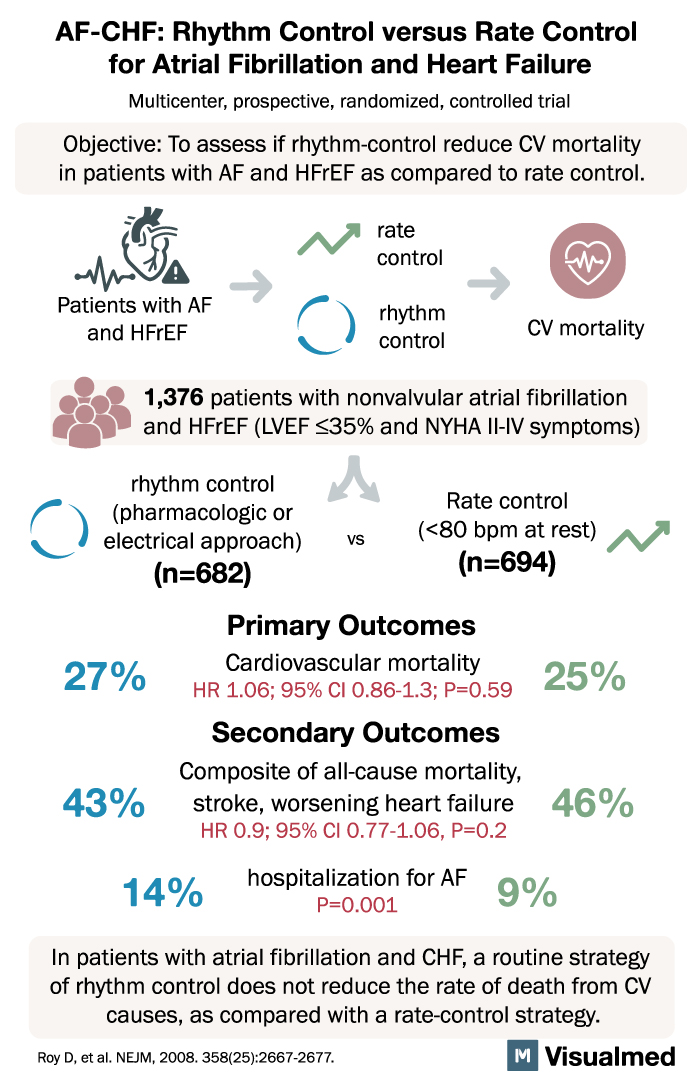
AF-CHF Trial Summary: Rhythm vs Rate Control for AFib and HF
AF-CHF: Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure Multicenter, prospective, randomized, controlled trial Objective: To assess if rhythm-control reduce CV mortality in patients with AF and HFrEF as compared to rate control. Patients with AF and … Read More