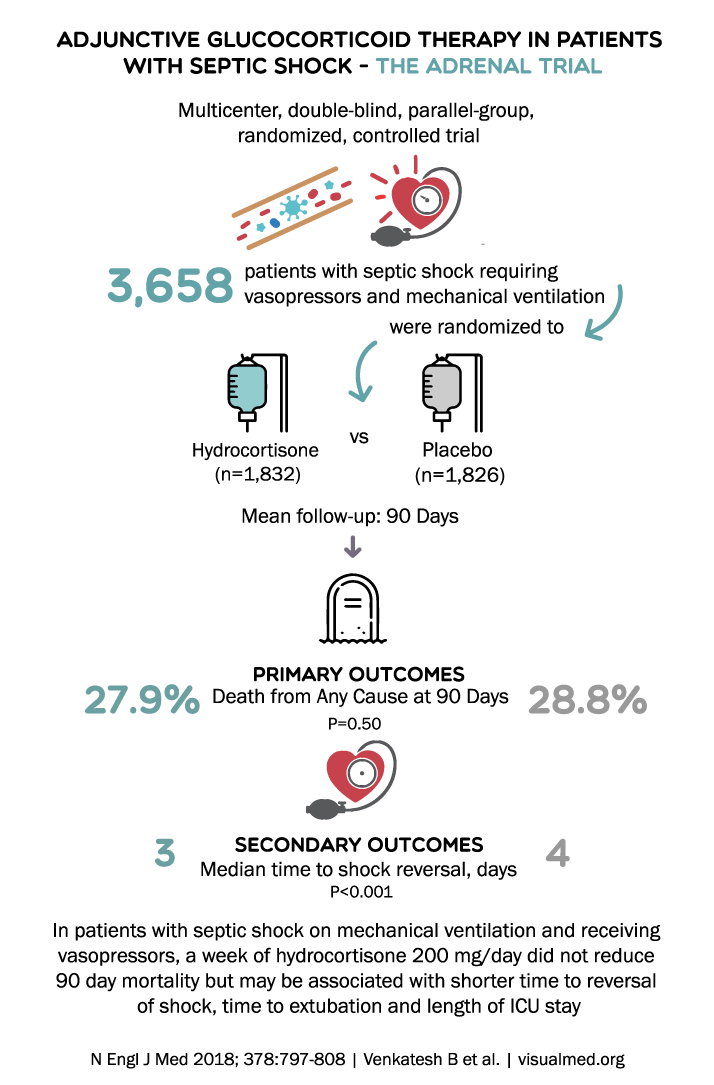
ADJUNCTIVE GLUCOCORTICOID THERAPY IN PATIENTS WITH SEPTIC SHOCK – THE ADRENAL TRIAL Multicenter, double-blind, parallel-group, randomized, controlled trial 3,658 patients with septic shock requiring vasopressors and mechanical ventilation were randomized to VS Hydrocortisone Placebo (n=1,832) (n=1,826) Mean follow-up: 90 Days … Read More
Blog
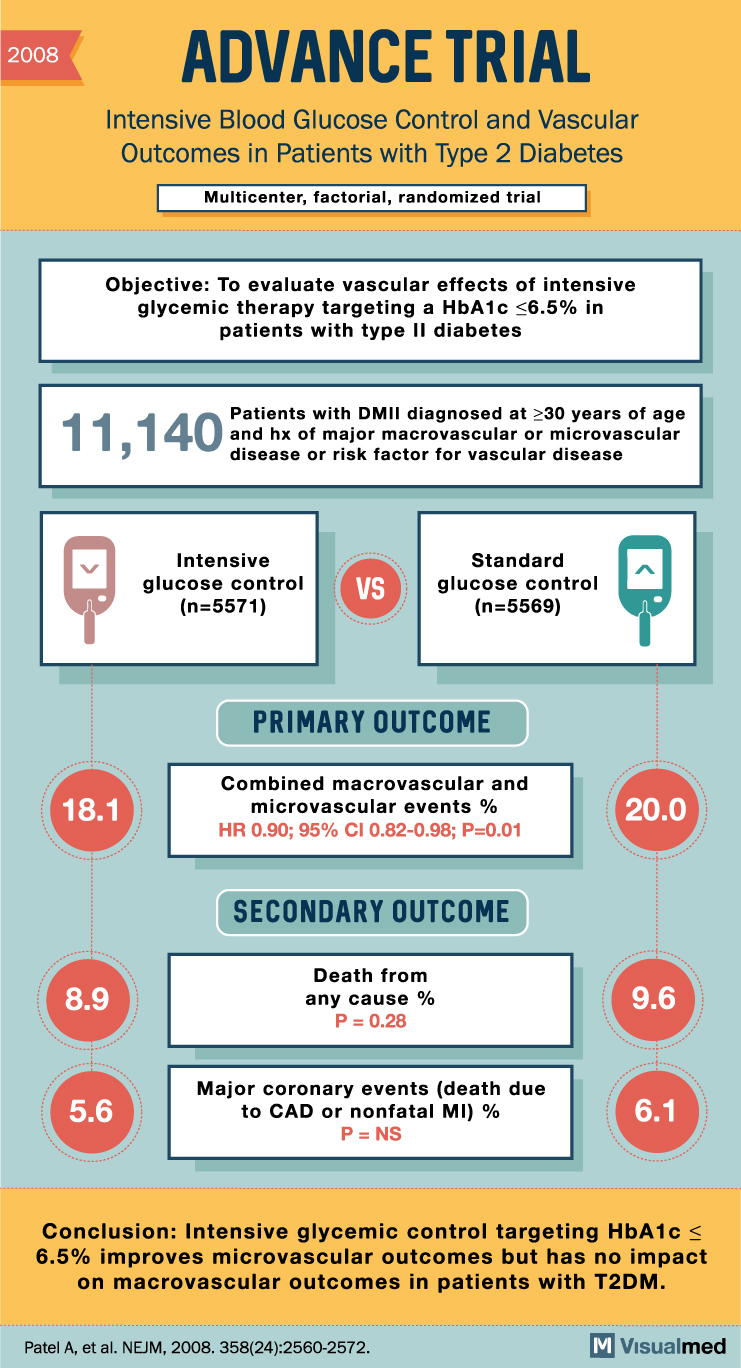
ADVANCE Trial Summary: Intensive Glucose Control in Diabetes Type 2
2008 ADVANCE TRIAL Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes Multicenter, factorial, randomized trial Objective: To evaluate vascular effects of intensive glycemic therapy targeting a HbA1c <6.5% in patients with type II diabetes 11,140 … Read More
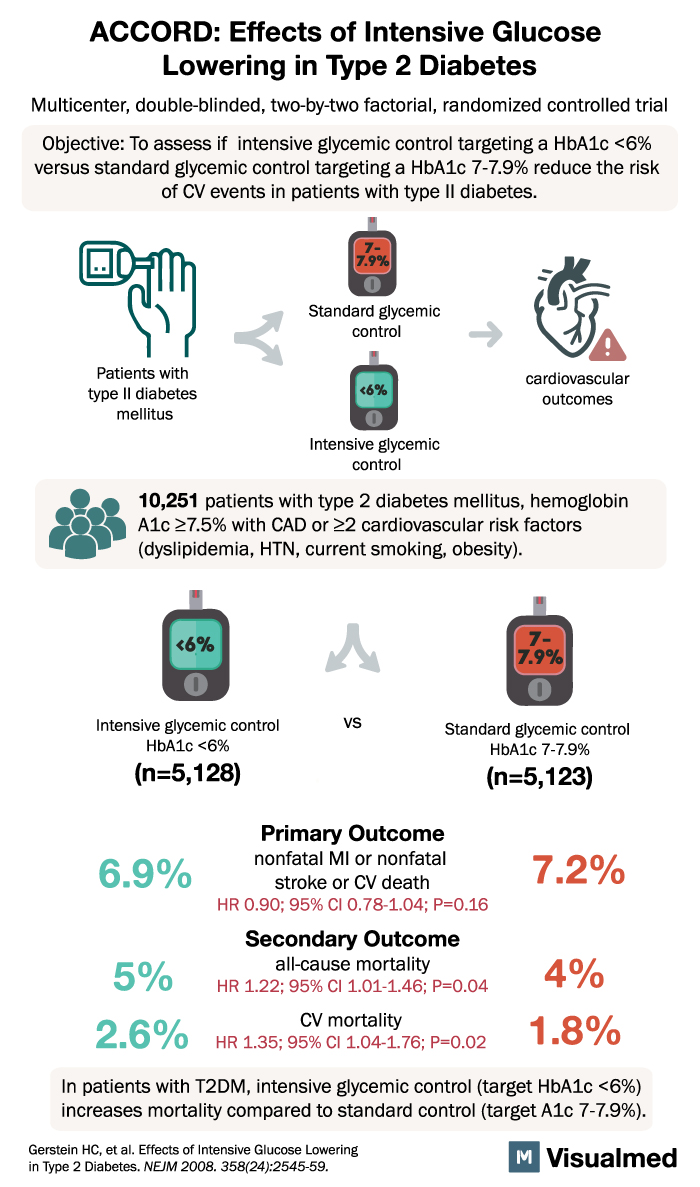
ACCORD Trial: Intensive Glucose Lowering in DM2
ACCORD: Effects of Intensive Glucose Lowering in Type 2 Diabetes Multicenter, double-blinded, two-by-two factorial, randomized controlled trial Objective: To assess if intensive glycemic control targeting a HbA1c <6% versus standard glycemic control targeting a HbA1c 7-7.9% reduce the risk of … Read More
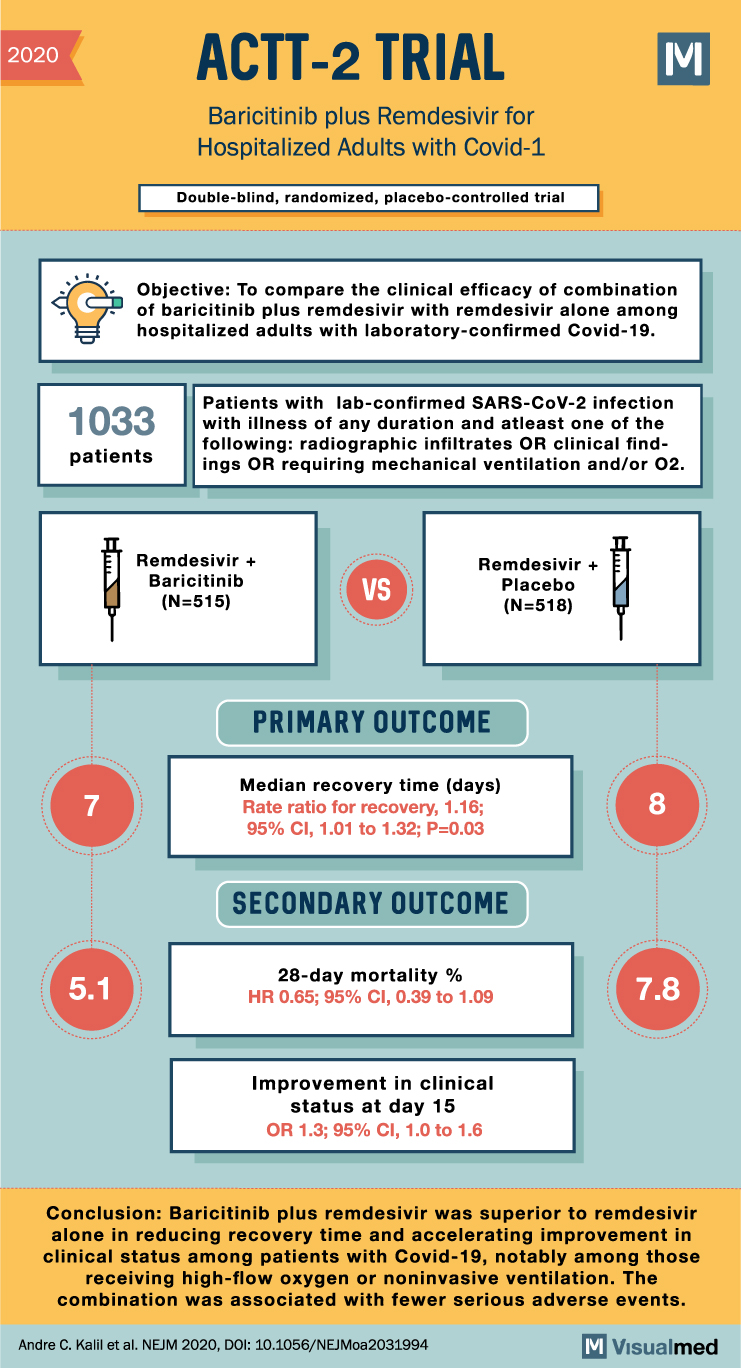
ACTT-2 Trial Summary: Baricitinib + Remdesivir in Covid-19
2020 ACTT-2 TRIAL Baricitinib plus Remdesivir for Hospitalized Adults with Covid-1 Double-blind, randomized, placebo-controlled trial M Objective: To compare the clinical efficacy of combination of baricitinib plus remdesivir with remdesivir alone among hospitalized adults with laboratory-confirmed Covid-19. 1033 patients Patients … Read More
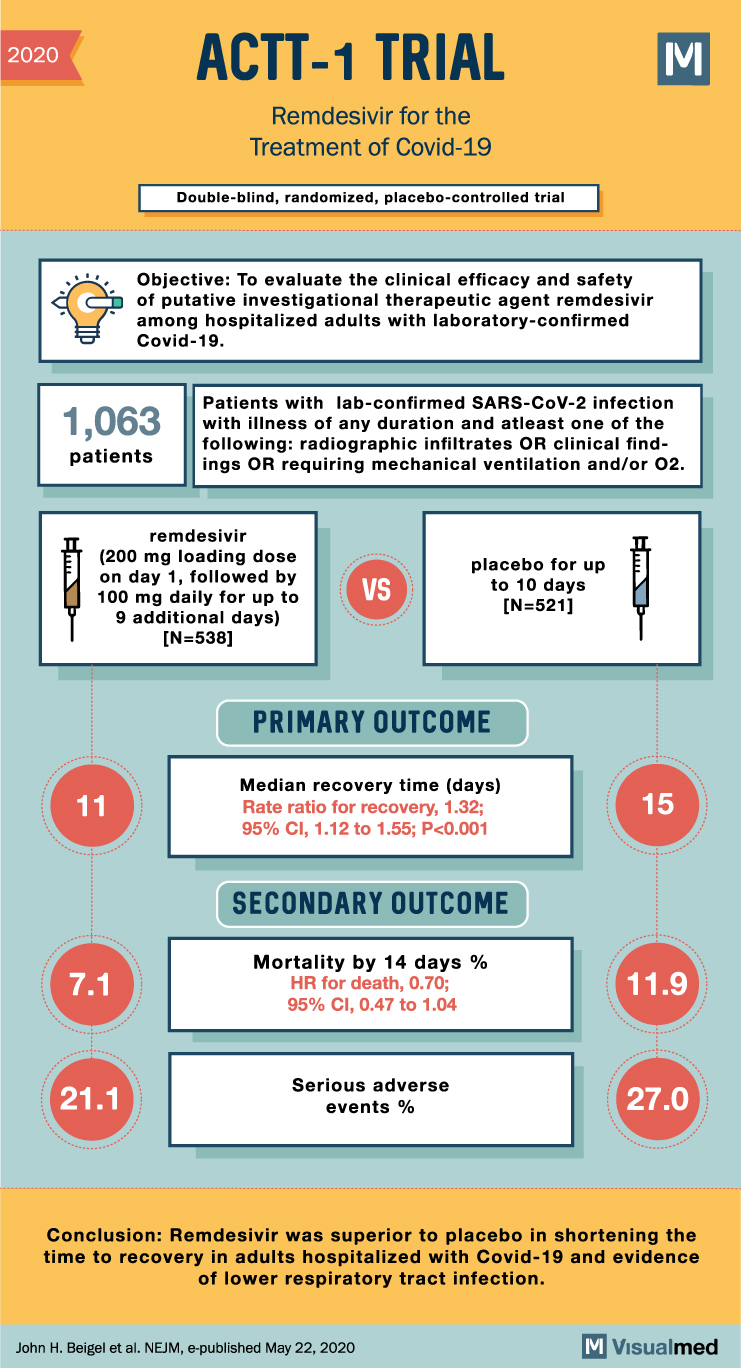
ACTT-1 Trial Summary: Remdesivir in Covid-19
2020 ACTT-1 TRIAL Remdesivir for the Treatment of Covid-19 Double-blind, randomized, placebo-controlled trial Objective: To evaluate the clinical efficacy and safety of putative investigational therapeutic agent remdesivir among hospitalized adults with laboratory-confirmed Covid-19. M Patients with lab-confirmed SARS-CoV-2 infection 1,063 … Read More
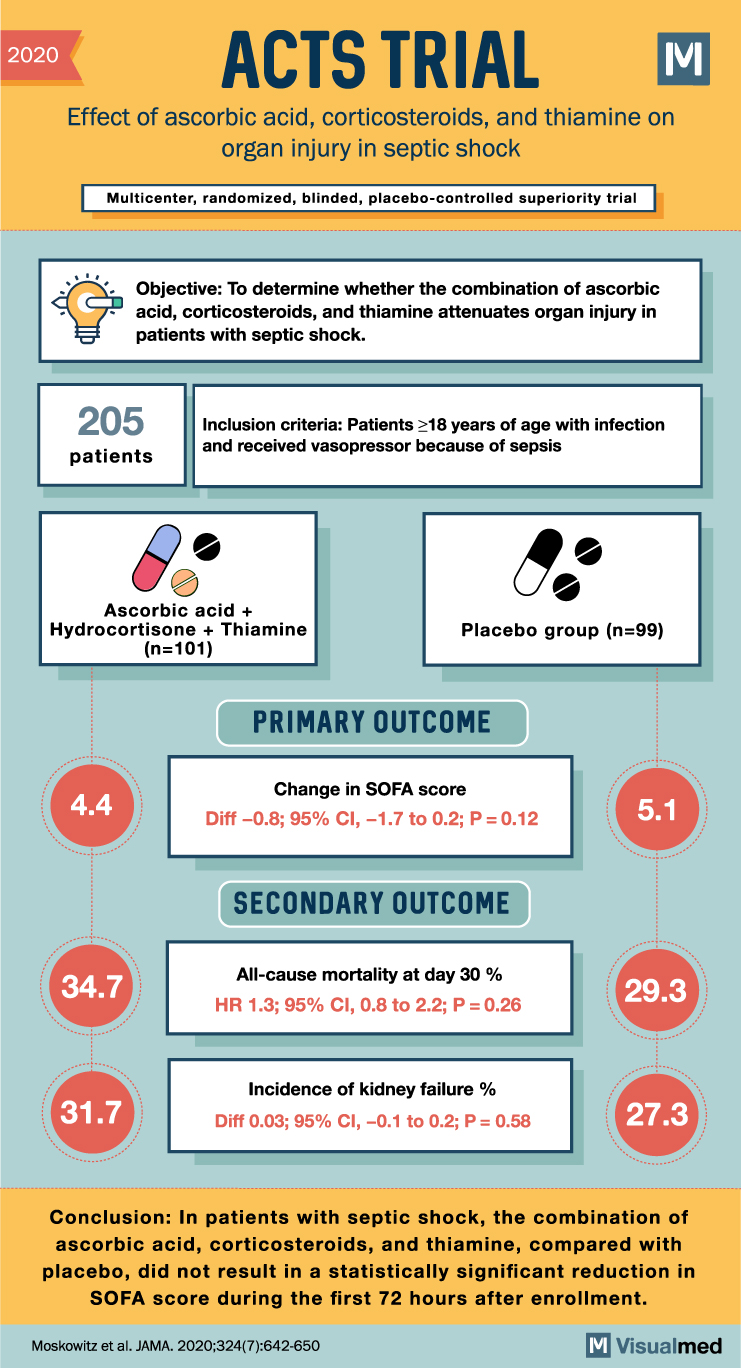
ACTS Trial: Ascorbic acid, Steroids and Thiamine in Septic Shock
2020 ACTS TRIAL Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock Multicenter, randomized, blinded, placebo-controlled superiority trial M Objective: To determine whether the combination of ascorbic acid, corticosteroids, and thiamine attenuates organ injury in patients … Read More
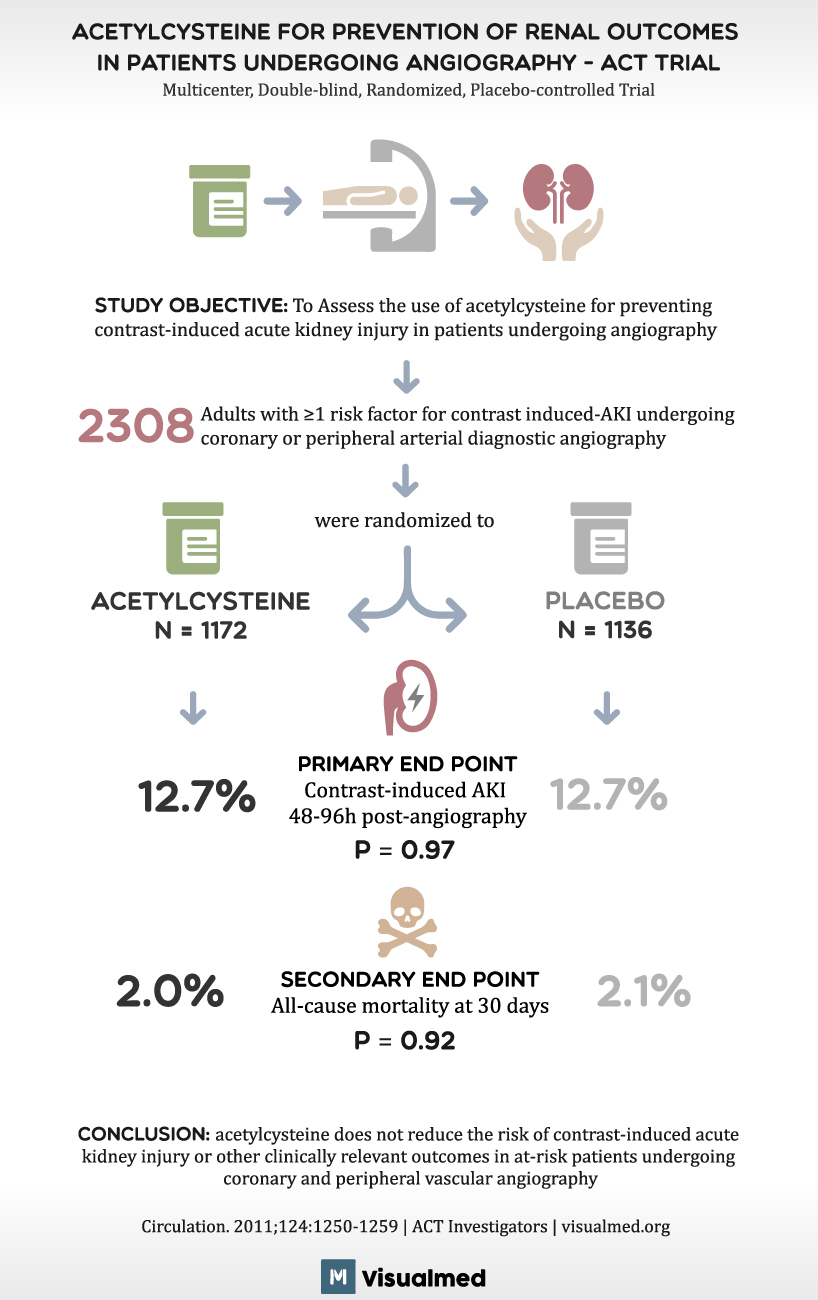
ACT Trial Summary: Acetylcysteine before Angiography
ACETYLCYSTEINE FOR PREVENTION OF RENAL OUTCOMES IN PATIENTS UNDERGOING ANGIOGRAPHY – ACT TRIAL Multicenter, Double-blind, Randomized, Placebo-controlled Trial STUDY OBJECTIVE: To Assess the use of acetylcysteine for preventing contrast-induced acute kidney injury in patients undergoing angiography 2308 Adults with ≥1 … Read More
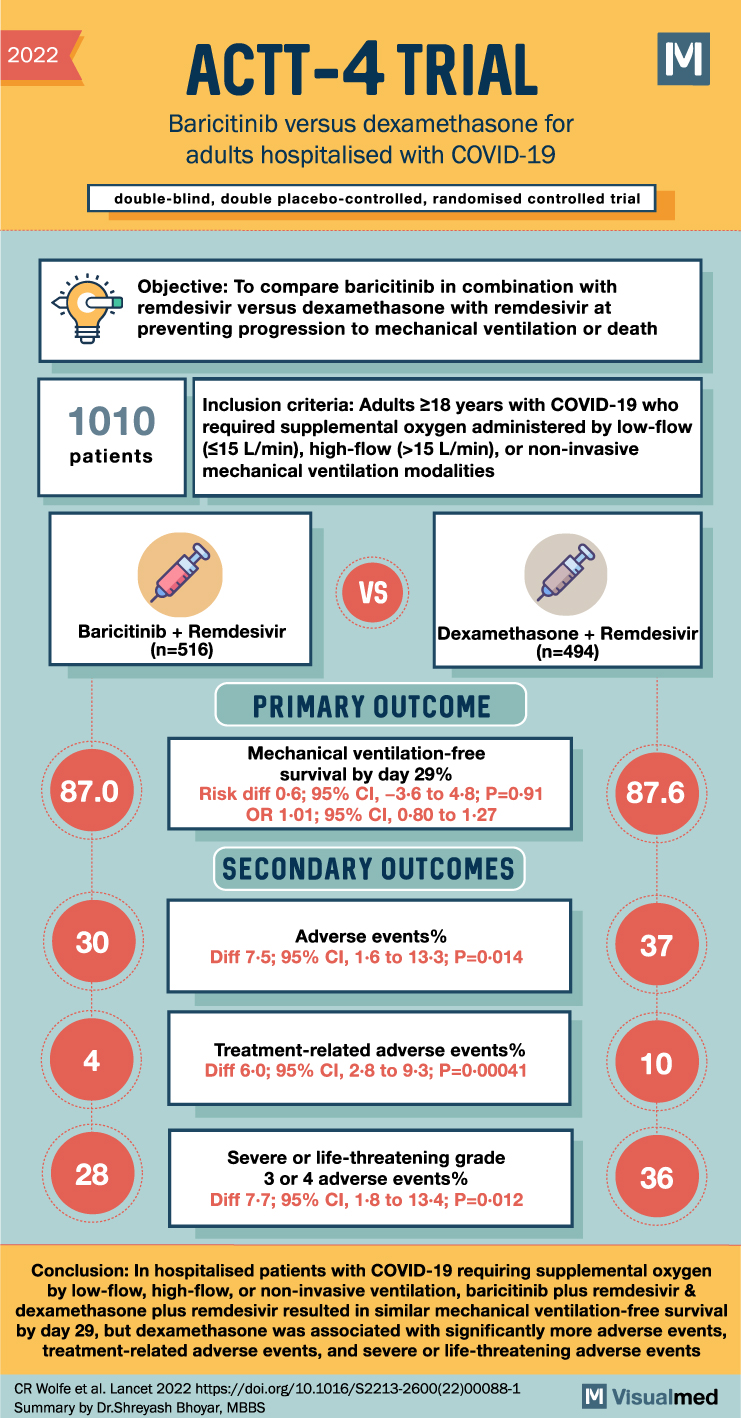
ACTT-4 Trial Summary: Baricitinib in Covid-19
2022 ACTT-4 TRIAL Baricitinib versus dexamethasone for adults hospitalised with COVID-19 double-blind, double placebo-controlled, randomised controlled trial M Objective: To compare baricitinib in combination with remdesivir versus dexamethasone with remdesivir at preventing progression to mechanical ventilation or death 1010 patients … Read More
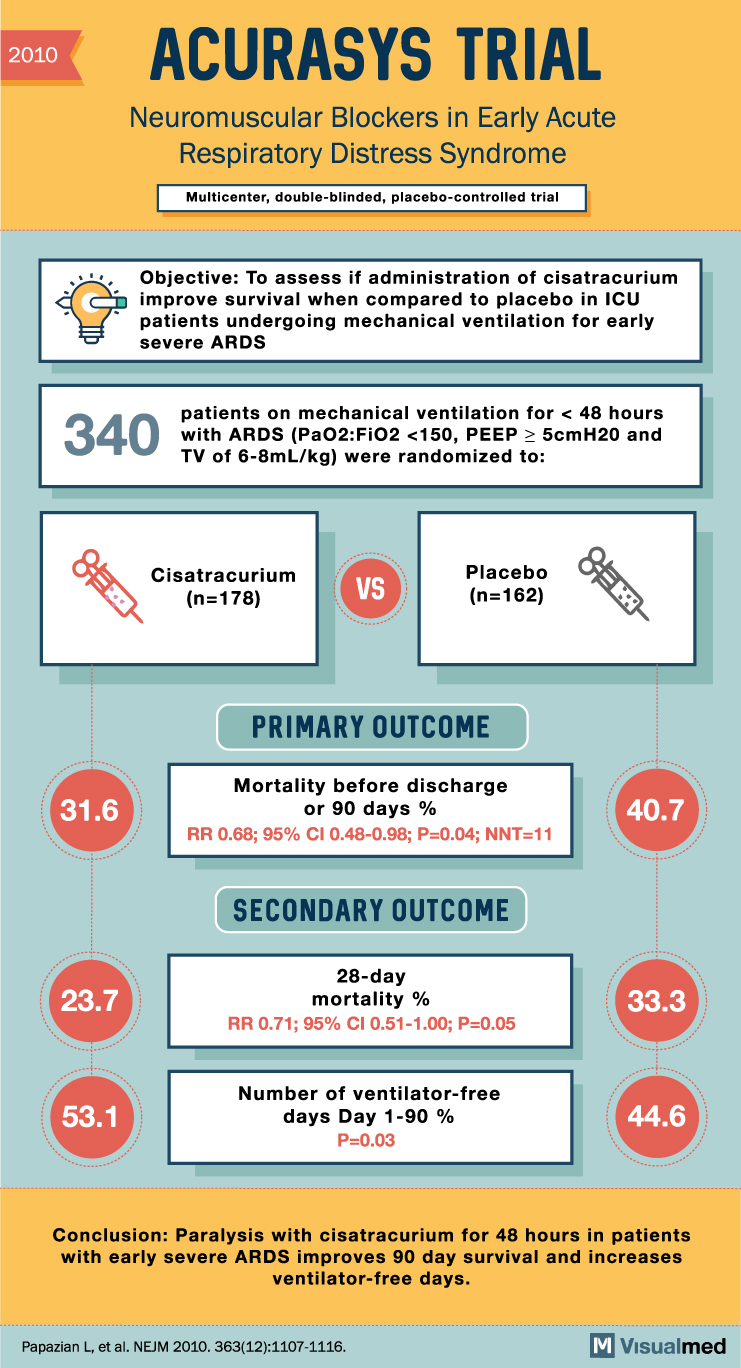
ACURASYS Trial Summary: NM blockers in ARDS
2010 ACURASYS TRIAL Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome Multicenter, double-blinded, placebo-controlled trial Objective: To assess if administration of cisatracurium improve survival when compared to placebo in ICU patients undergoing mechanical ventilation for early severe ARDS 340 patients … Read More
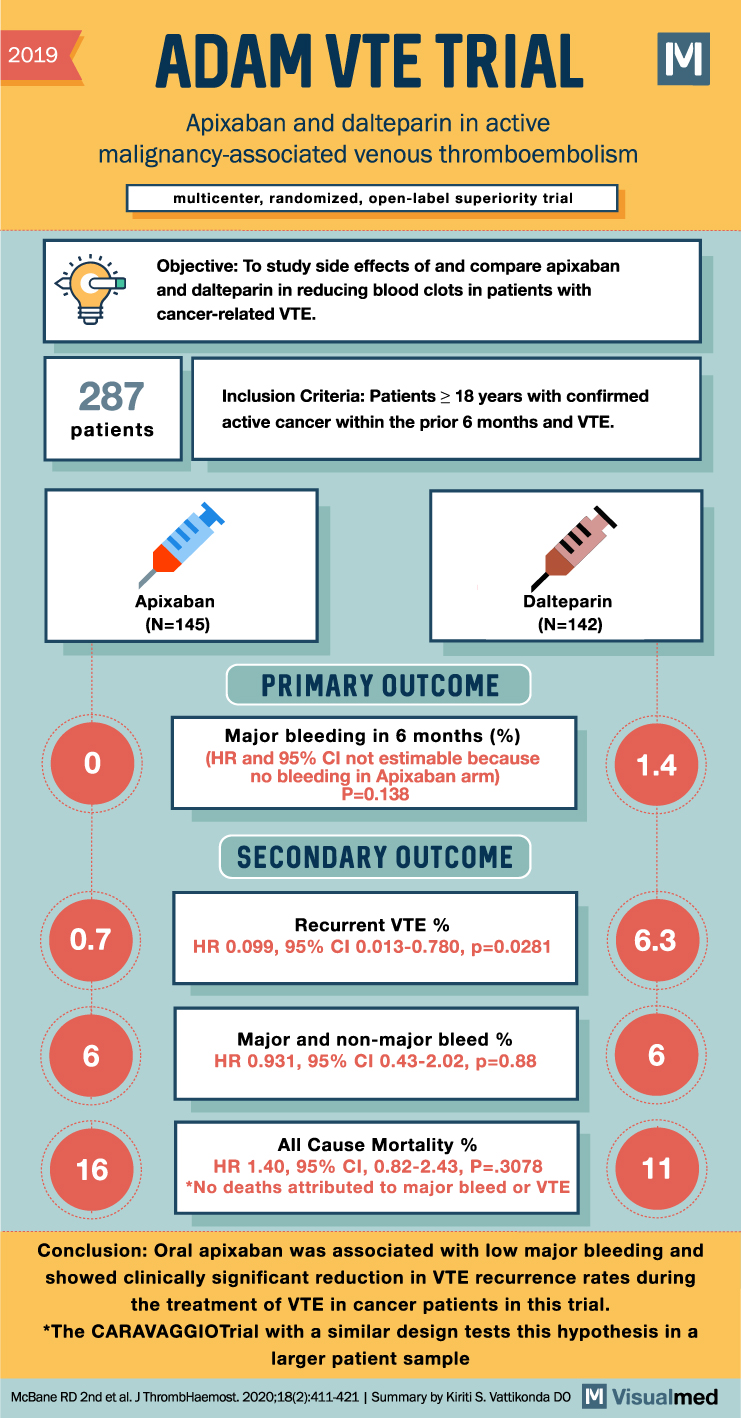
ADAM VTE Trial Summary: Apixaban in Cancer-associated VTE
2019 ADAM VTE TRIAL Apixaban and dalteparin in active malignancy-associated venous thromboembolism multicenter, randomized, open-label superiority trial 287 patients 0 Objective: To study side effects of and compare apixaban and dalteparin in reducing blood clots in patients with cancer-related VTE. … Read More