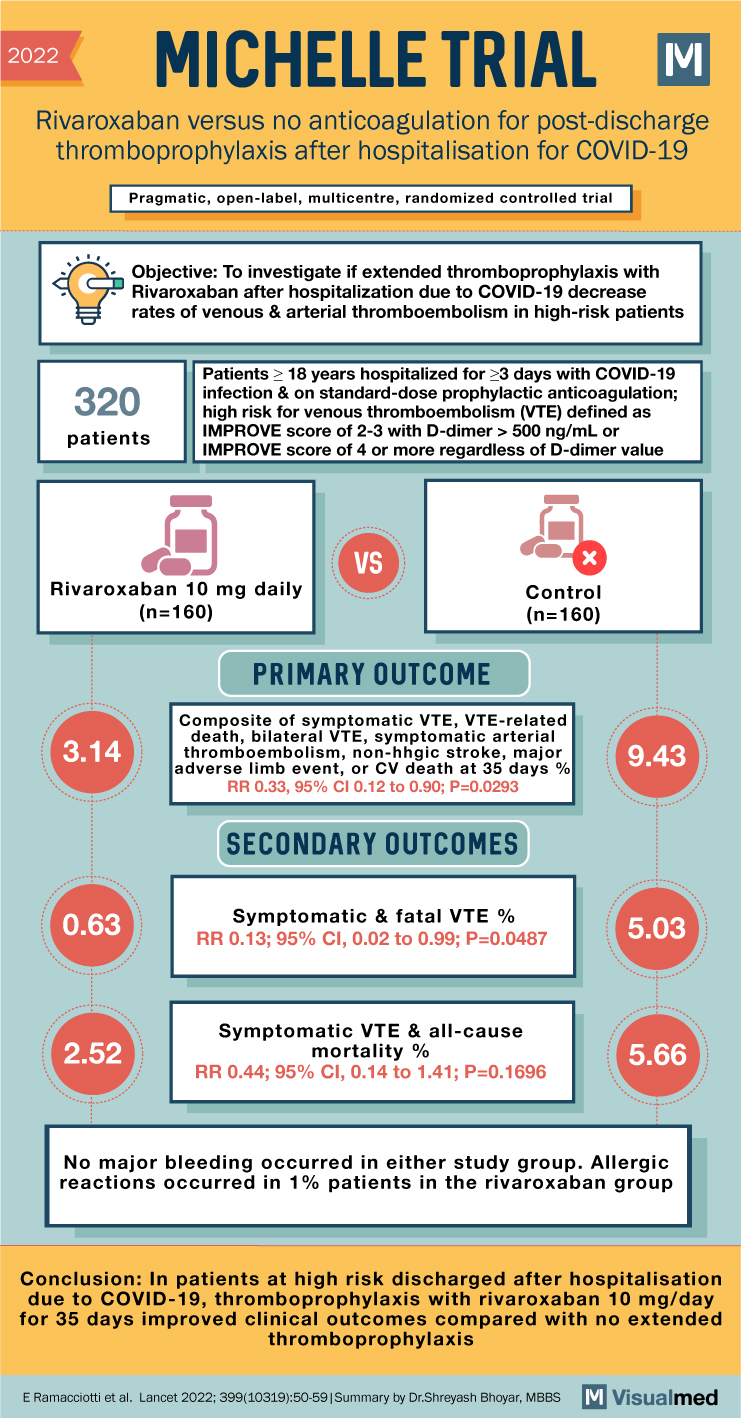
2022 MICHELLE TRIAL Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 Pragmatic, open-label, multicentre, randomized controlled trial Objective: To investigate if extended thromboprophylaxis with Rivaroxaban after hospitalization due to COVID-19 decrease rates of venous & arterial thromboembolism … Read More
Blog
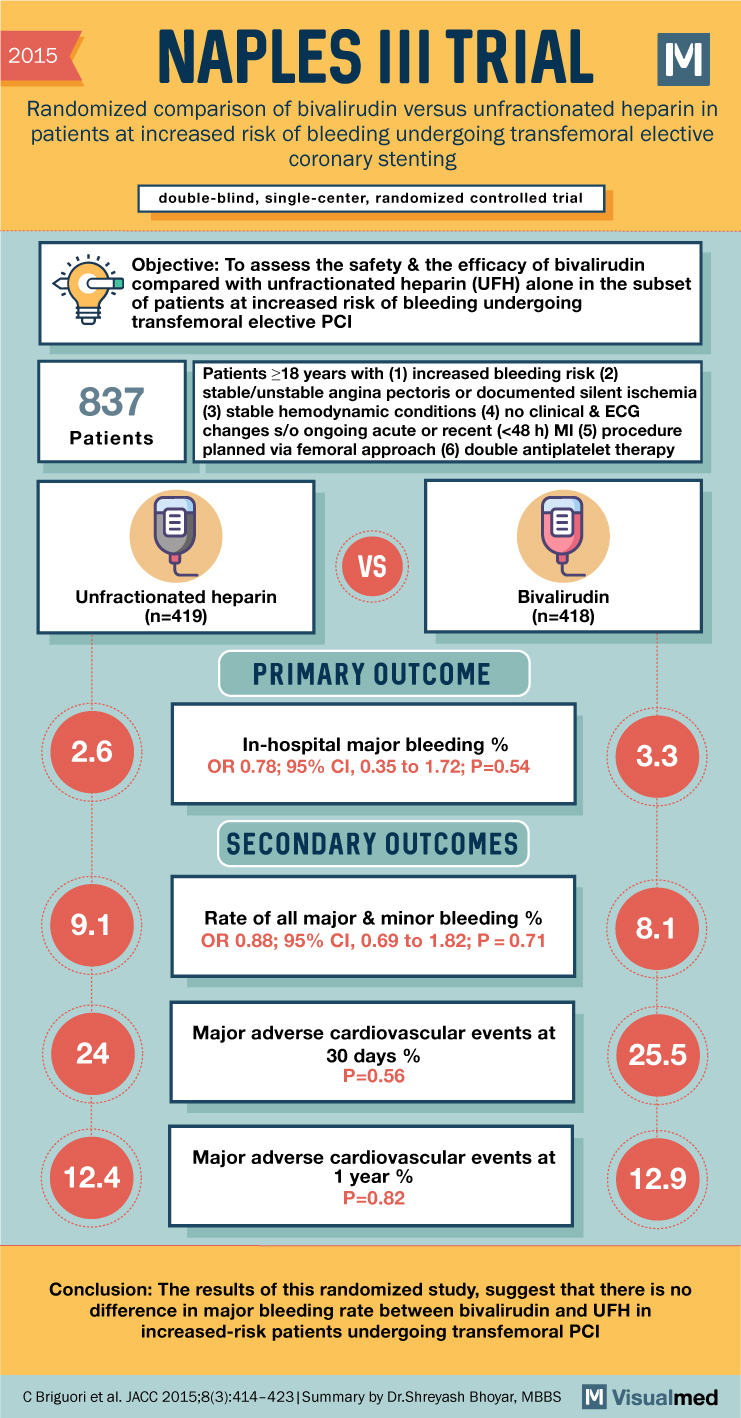
NAPLES III Trial Summary: Bivalirudin in Patients with High Risk of Bleeding Undergoing LHC
2015 NAPLES III TRIAL Randomized comparison of bivalirudin versus unfractionated heparin in patients at increased risk of bleeding undergoing transfemoral elective coronary stenting double-blind, single-center, randomized controlled trial Objective: To assess the safety & the efficacy of bivalirudin compared with … Read More
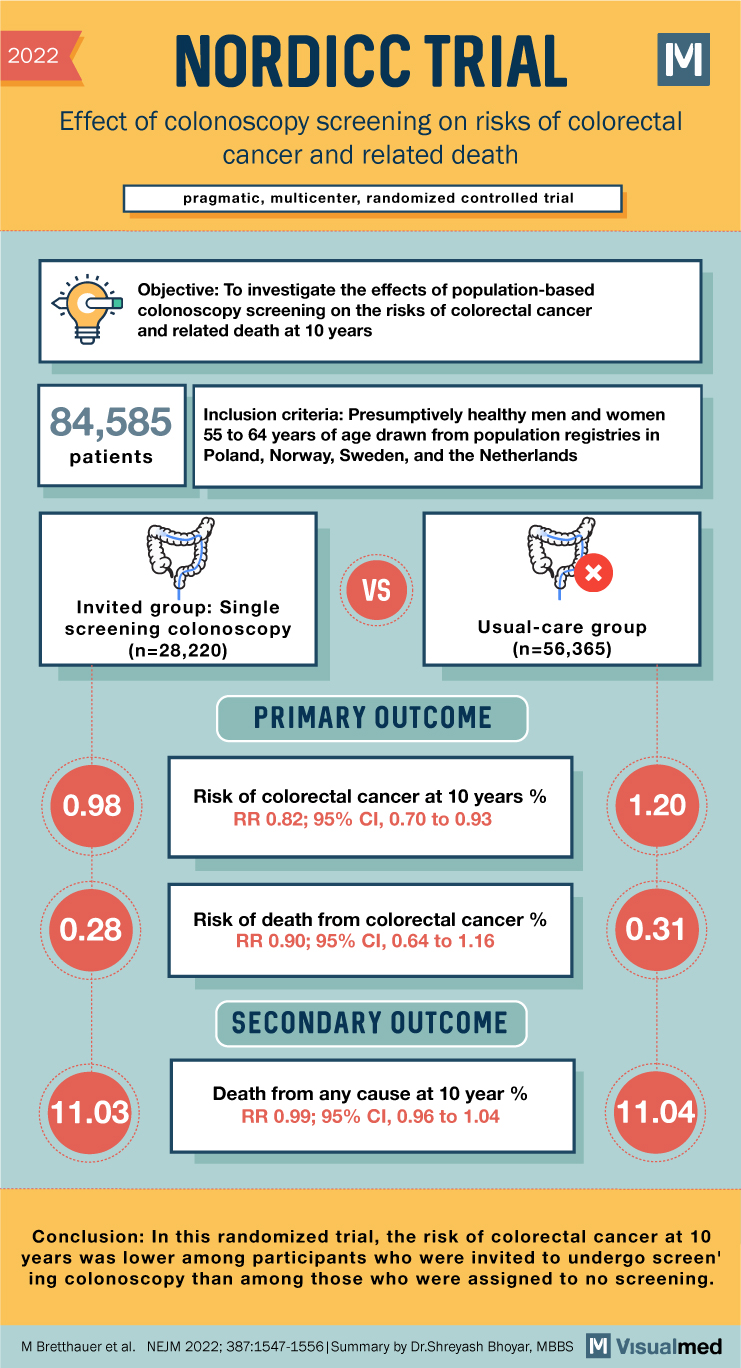
NORDICC Trial Summary: Colonoscopy for Colorectal Cancer Screening
2022 NORDICC TRIAL Effect of colonoscopy screening on risks of colorectal cancer and related death pragmatic, multicenter, randomized controlled trial Objective: To investigate the effects of population-based colonoscopy screening on the risks of colorectal cancer and related death at 10 … Read More
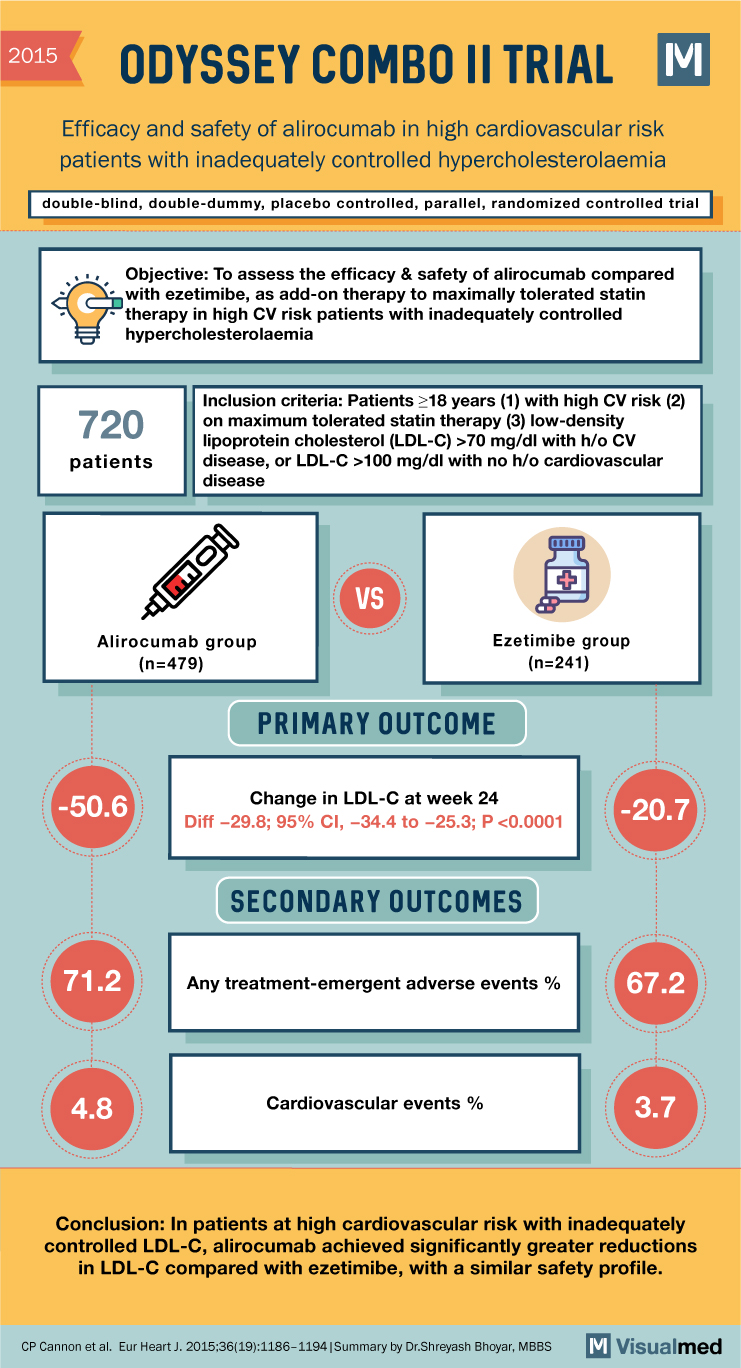
ODYSSEY COMBO II Trial Summary: Alirocumab in High CV risk Patients with Hypercholesterolemia
2015 ODYSSEY COMBO II TRIAL Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia double-blind, double-dummy, placebo controlled, parallel, randomized controlled trial Objective: To assess the efficacy & safety of alirocumab compared with therapy to … Read More
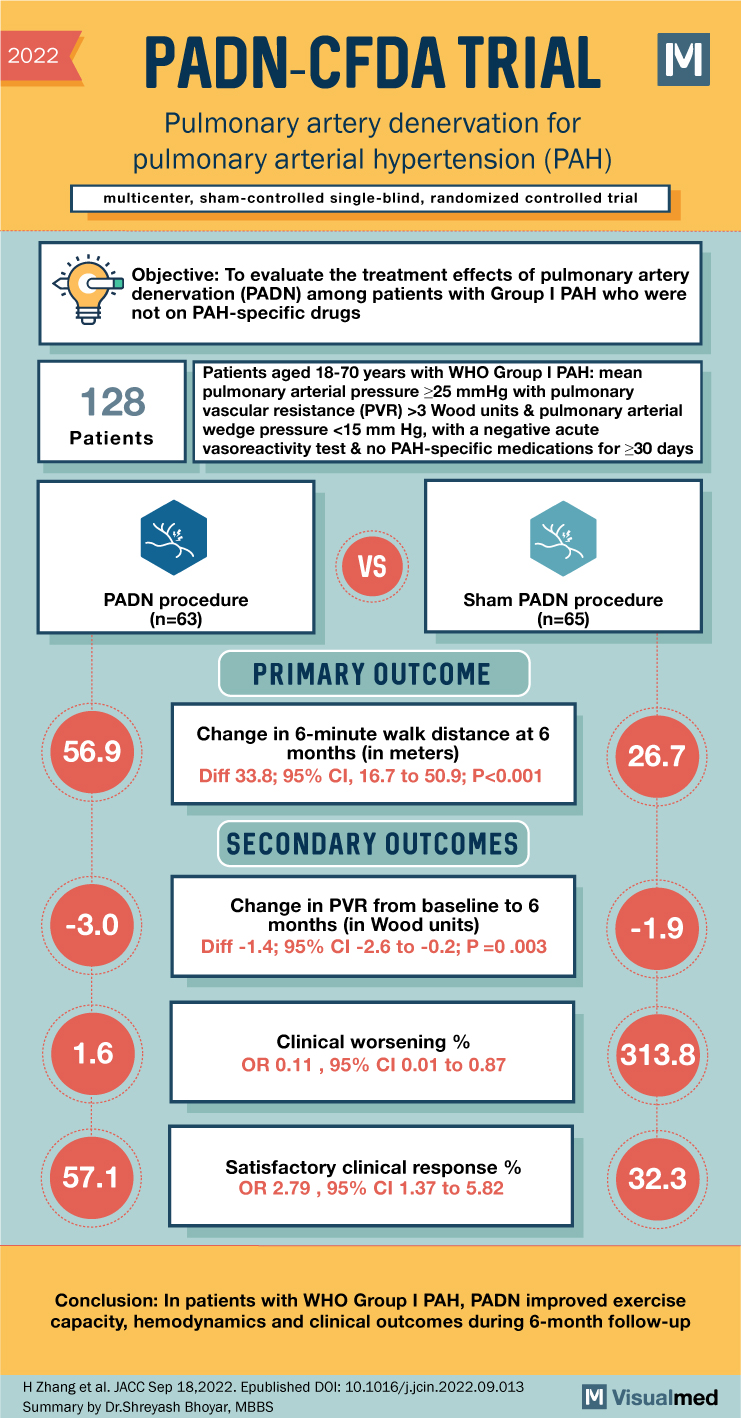
PADN-CFDA Trial Summary: PA Denervation for PAH
2022 PADN-CFDA TRIAL Pulmonary artery denervation for pulmonary arterial hypertension (PAH) multicenter, sham-controlled single-blind, randomized controlled trial Objective: To evaluate the treatment effects of pulmonary artery denervation (PADN) among patients with Group I PAH who were not on PAH-specific drugs … Read More
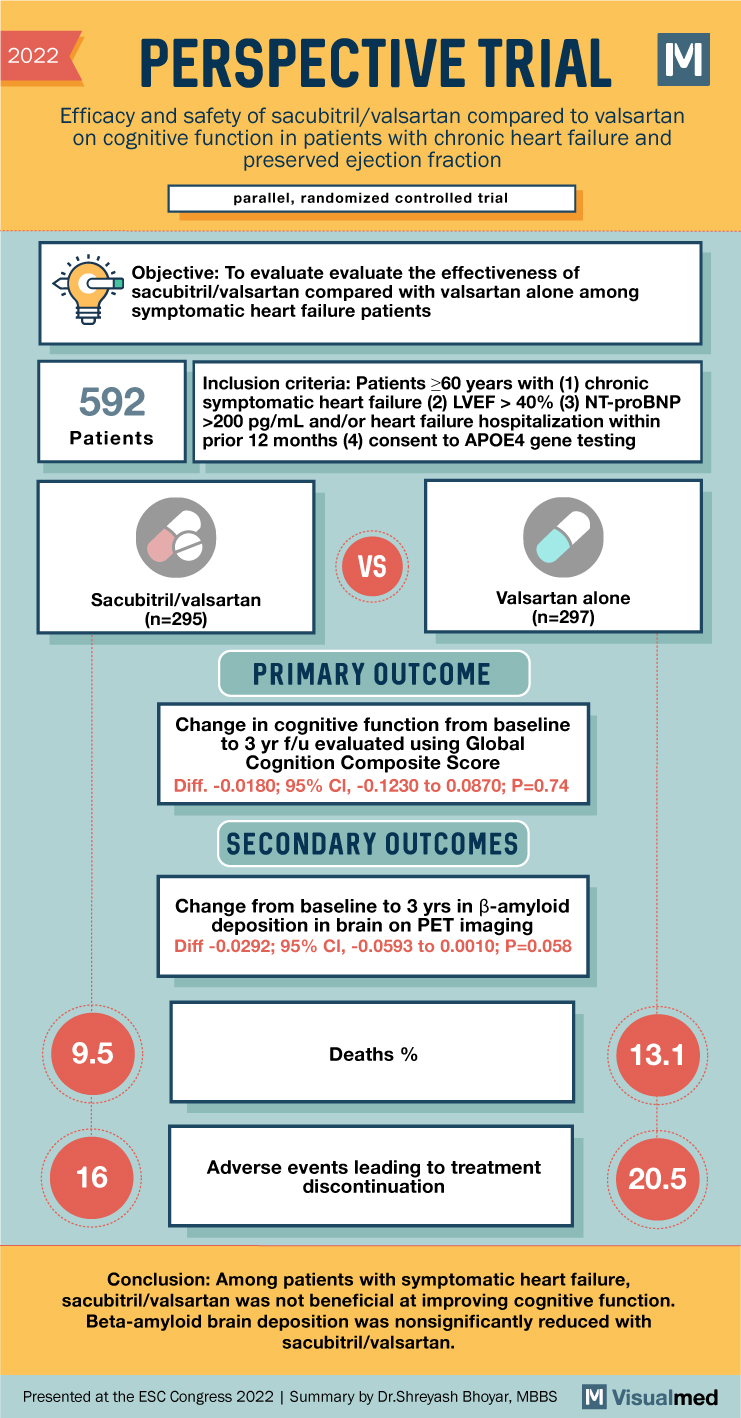
PERSPECTIVE Trial Summary: Sacubitril/valsartan Effect on Cognition in HFpEF
2022 PERSPECTIVE TRIAL M Efficacy and safety of sacubitril/valsartan compared to valsartan on cognitive function in patients with chronic heart failure and preserved ejection fraction parallel, randomized controlled trial Objective: To evaluate the effectiveness of sacubitril/valsartan compared with valsartan alone … Read More
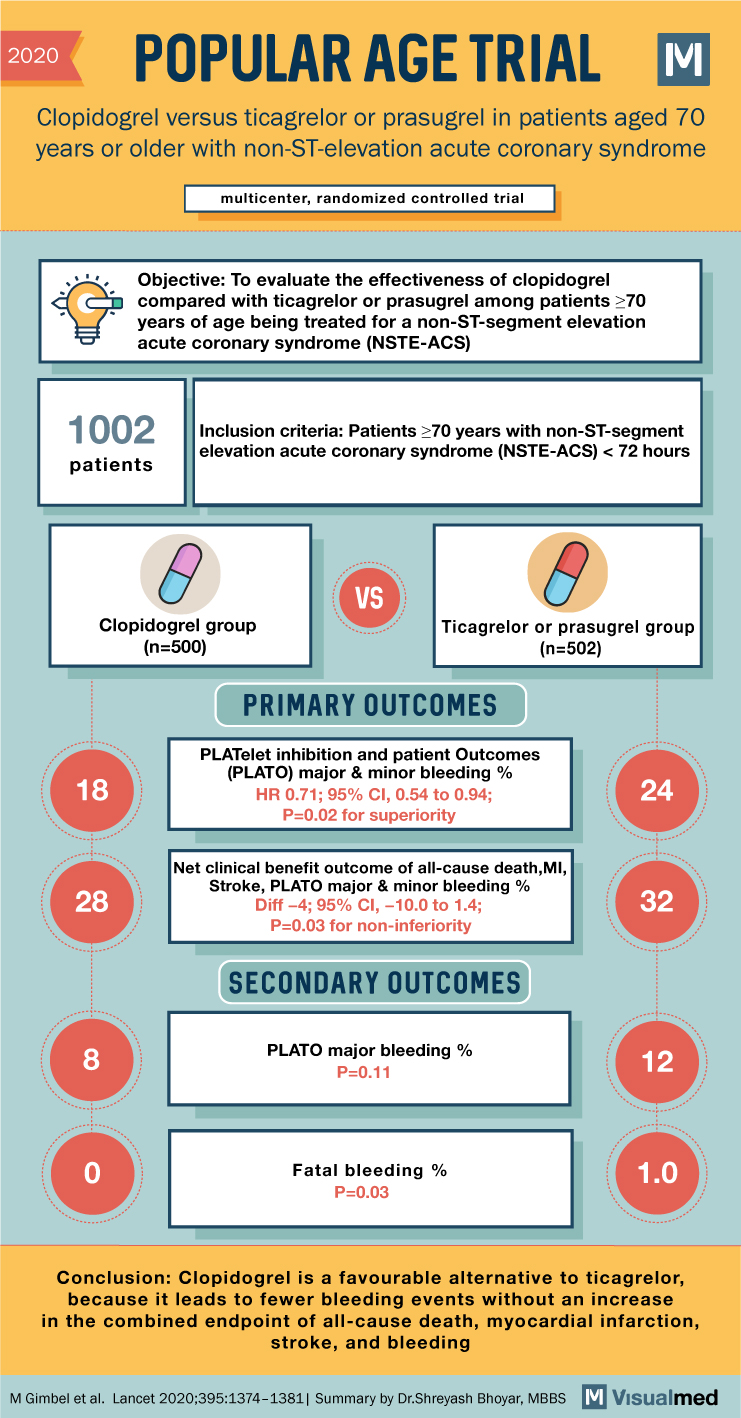
POPular AGE Trial Summary: Antiplatelet agent in NSTEMI
2020 POPULAR AGE TRIAL Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome multicenter, randomized controlled trial Objective: To evaluate the effectiveness of clopidogrel compared with ticagrelor or prasugrel among patients ≥70 … Read More
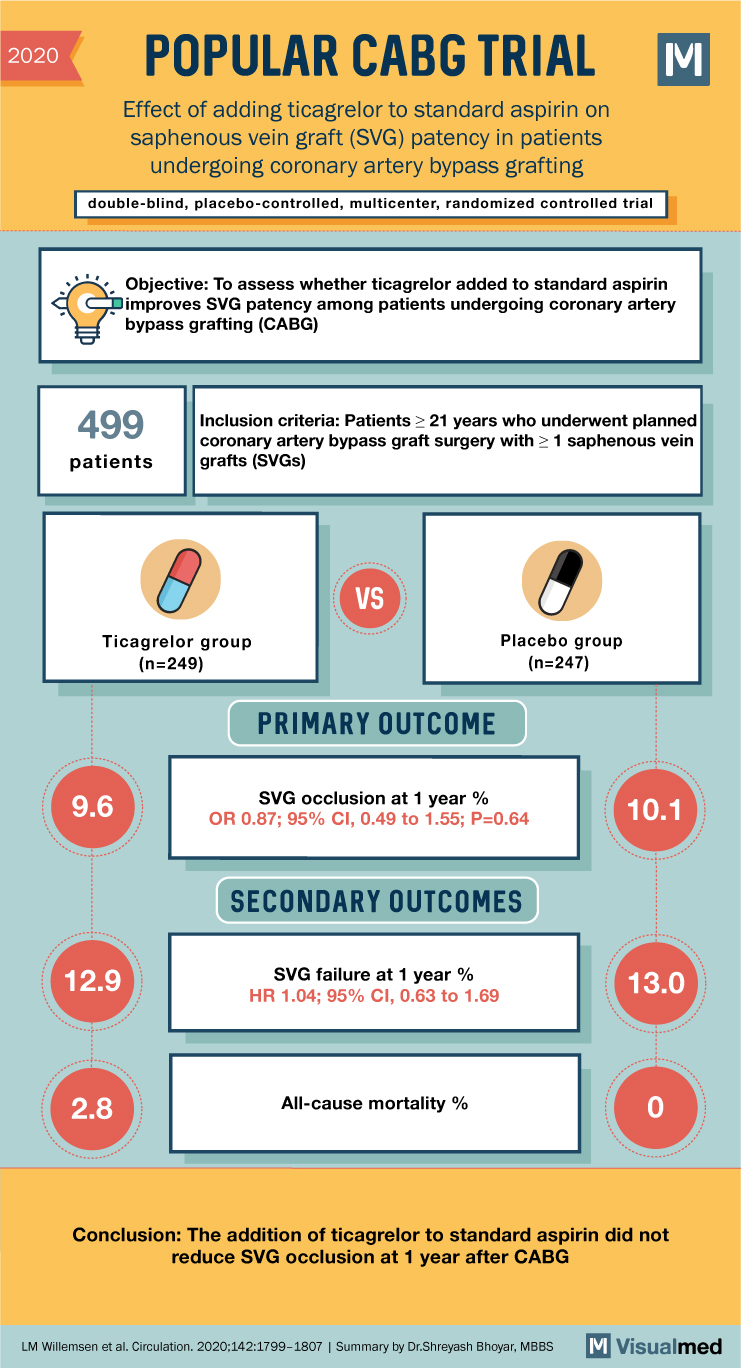
POPULAR CABG Trial Summary: Ticagrelor in CABG
2020 POPULAR CABG TRIAL Effect of adding ticagrelor to standard aspirin on saphenous vein graft (SVG) patency in patients undergoing coronary artery bypass grafting double-blind, placebo-controlled, multicenter, randomized controlled trial M Objective: To assess whether ticagrelor added to standard aspirin … Read More
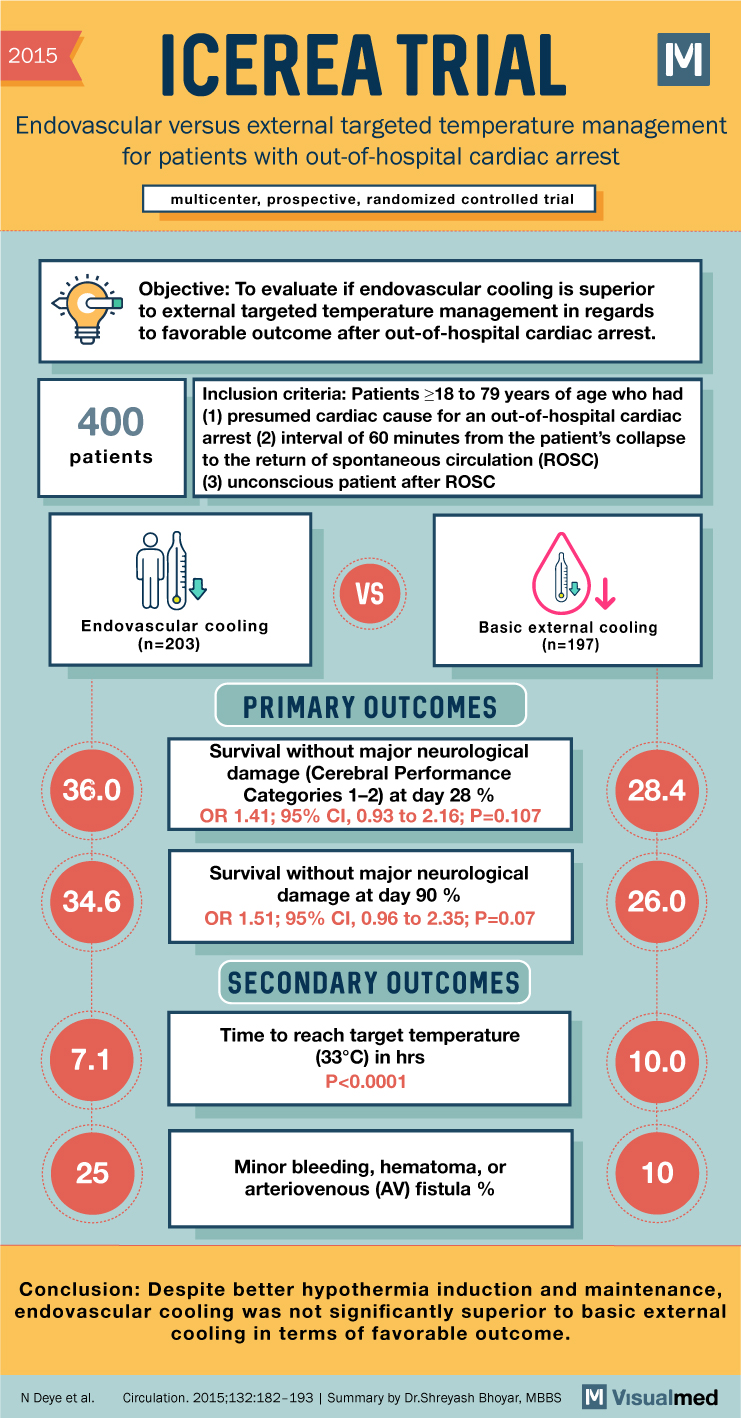
ICEREA Trial Summary: Endovascular Versus External TTM in Cardiac Arrest
2015 ICEREA TRIAL Endovascular versus external targeted temperature management for patients with out-of-hospital cardiac arrest multicenter, prospective, randomized controlled trial Objective: To evaluate if endovascular cooling is superior to external targeted temperature management in regards to favorable outcome after out-of-hospital … Read More
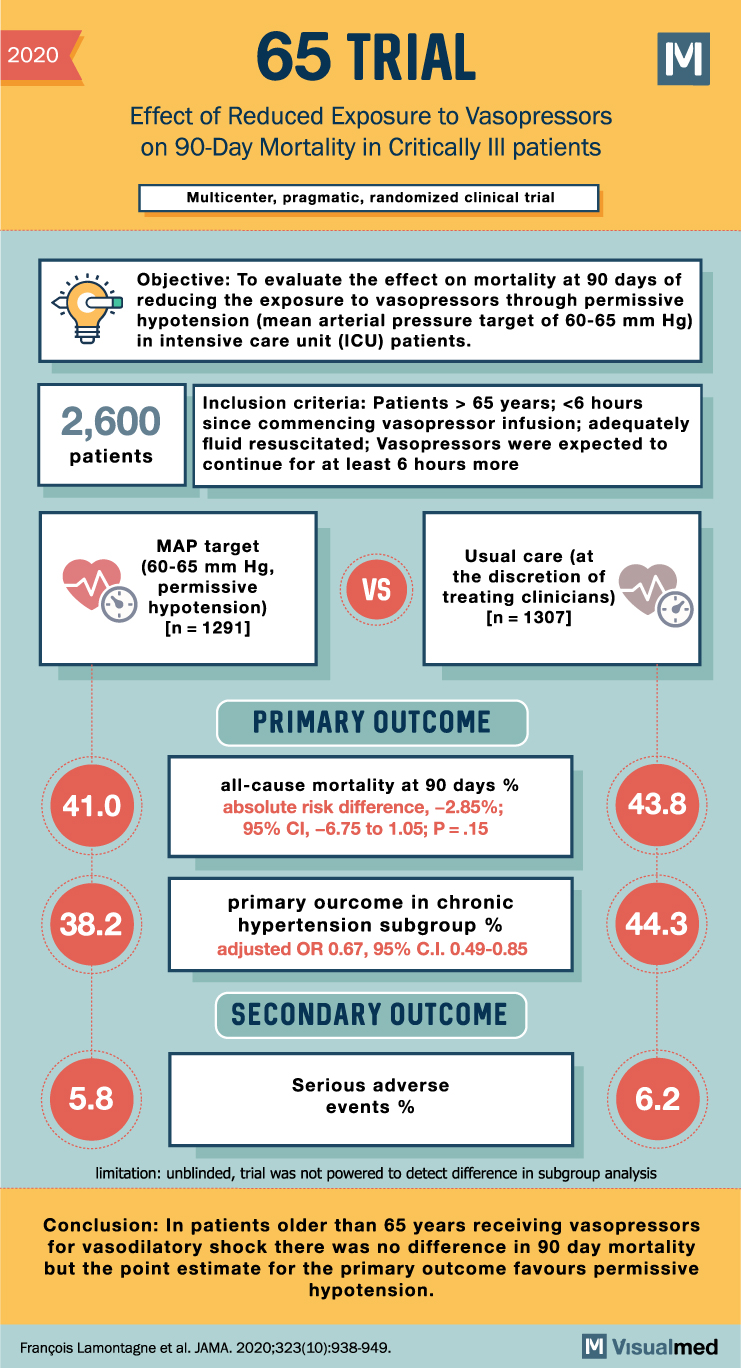
65 Trial Summary: Vasopressors in Older Critically Ill Patients
2020 65 TRIAL Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Critically III patients Multicenter, pragmatic, randomized clinical trial Objective: To evaluate the effect on mortality at 90 days of reducing the exposure to vasopressors through permissive hypotension … Read More